Coating structure of medical biodegradable zinc alloy stent and preparation method thereof
A zinc alloy and drug coating technology, applied in metal material coating technology, coating, medical science and other directions, can solve the problems of uncontrollable degradation rate, uncontrollable degradation rate and low mechanical strength of pure zinc and zinc alloy materials , to achieve the effect of improving pitting corrosion defects, good biocompatibility, and no toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
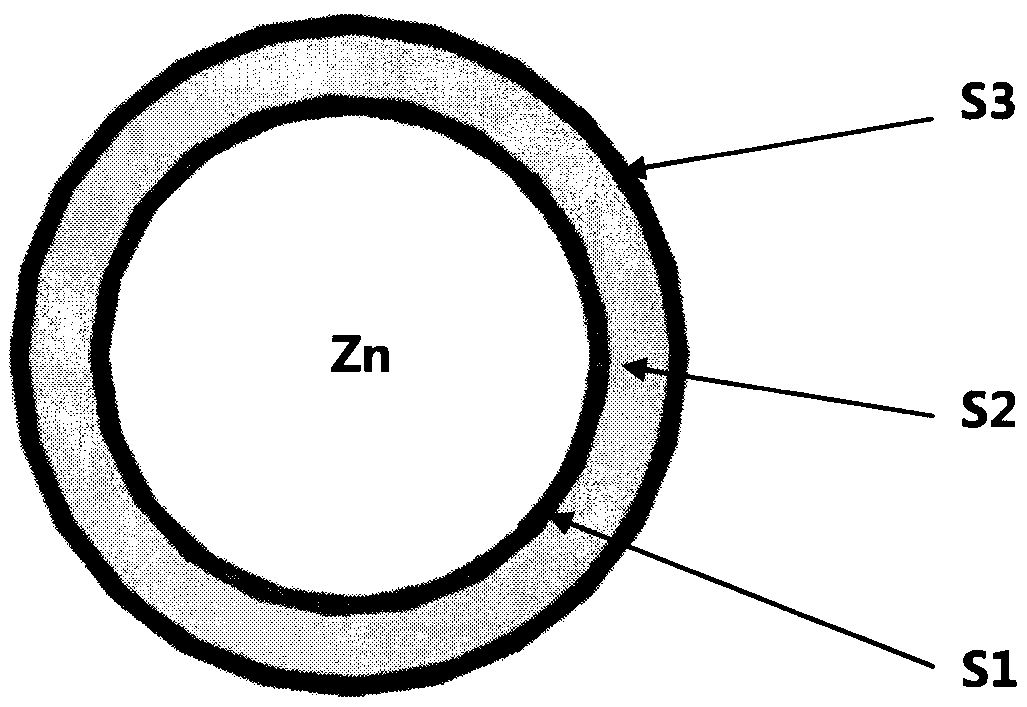
[0030] Example 1, oxide-coated stent A1:
[0031] Take the polished zinc alloy bracket and put it into a vacuum tube furnace, evacuate it to -0.1Mpa, then fill it with high-purity oxygen to 0.12Mpa; then heat the vacuum tube furnace to 200°C and keep it for 2 hours; finally cool it naturally and release it slowly Oxygen to normal pressure to obtain S1 oxide-coated stent A1.
Embodiment 2
[0032] Embodiment 2, composite coated stent A2:
[0033] Take the polished zinc alloy bracket and put it into a vacuum tube furnace, evacuate it to -0.1Mpa, then fill it with high-purity oxygen to 0.12Mpa; then heat the vacuum tube furnace to 200°C and keep it for 2 hours; finally cool it naturally and release it slowly Oxygen to normal pressure, the S1 oxide-coated stent was obtained.
[0034] Take polylactic acid (PLA) with a molecular weight of 80,000 and polytrimethylene carbonate (PTMC) with a molecular weight of 30,000 and mix and dissolve in methylene chloride. The mass ratio of PLA: PTMC is 2: 3. The mass concentration is 0.5%; then use ultrasonic atomization spraying to coat the mixed solution on the stent containing the S1 oxide coating; finally put the coated stent in a vacuum drying oven, set the temperature at 45°C for 36h, and pump Vacuum to -0.1Mpa. After drying, the composite coated stent A2 containing S1 and S2 is obtained.
Embodiment 3
[0035] Embodiment 3, composite coated stent A3:
[0036] S1, S2 composite coating stent preparation method, same as embodiment 2
[0037] First get 0.1g rapamycin and 0.35g molecular weight polylactic acid-glycolic acid copolymer (PLGA) of 10,000 and dissolve in acetone, the mass concentration of mixed solution is 1.1%; The solution was coated on the composite coating stent containing S1 and S2; finally, the coated stent was placed in a vacuum drying oven, the temperature was set at 40°C, the time was 24h, and the vacuum was pumped to -0.1Mpa. After drying, the composite coating stent A3 containing S1, S2, and S3 is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| coating thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com