Method for separating phellodendrine glucuronic acid conjugate from urine
A technology of aldehyde acid conjugate and glucuronide is applied in the field of separation of metabolites of Phellodendron alkaloids in vivo, which can solve the problems of less Phellodendron alkaloids and no research report on the metabolism, and achieves the effects of simple operation, low cost and high efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the separation of Phellodendronine glucuronide
[0031] Eighteen SD rats were intragastrically given the solution of cortex base at the dose of 80 mg / kg cortex base, once a day, collected urine samples from 0 to 24 hours, and administered continuously for two weeks. The collected urine samples were combined and centrifuged to remove the residue, and stored at -80°C for separation.
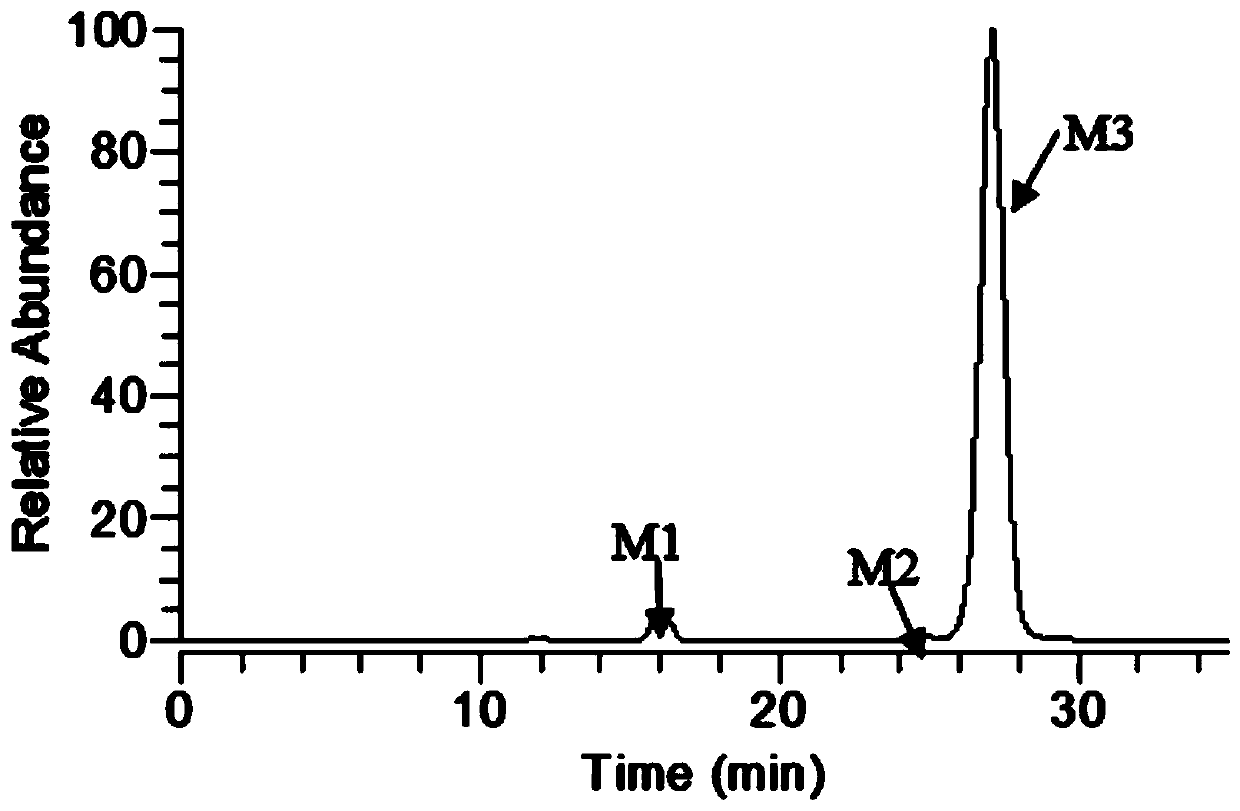
[0032] During the separation process, each elution fraction was analyzed using the established LC-MS n analysis by the method, and distinguish the metabolites M1, M2, and M3 according to the peak time, such as figure 1 . M1 is phellodine-2,11-O-D-glucuronide, M2 is phellodine-2-O-D-glucuronide, and M3 is phellodine-11-O-D-glucuronide.
[0033] 1.1 Preliminary separation of AB-8 macroporous adsorption resin:
[0034] A total of 4.5 L of urine samples were collected. Firstly, the urine samples were suction-filtered to remove impurities and precipitates, and then treated with ...
Embodiment 2
[0045] Embodiment 2: the preparation of phellodine glucuronide:
[0046] Preparation conditions for metabolite M1: use Phenomenes Polar-RP-80A semi-preparative column (10×250mm, 4μm), flow rate is 4mL / min; mobile phase is methanol: ultrapure water (5:95), injection time is 15min, the detection wavelength is 234nm, the injection volume is 80μL, the collection time is 4-4.5min, RT=4.25min, after preparation, all collected solutions are combined and concentrated to dryness, dissolved in 5mL ultrapure water, stored at -80℃, and finally used After freeze-drying, 10 mg of off-white powder was obtained.
[0047] Metabolite M2 preparation conditions: Phenomenes Polar-RP-80A semi-preparative column (10×250mm, 4μm), flow rate is 4mL / min; mobile phase is methanol: ultrapure water (25:75), and the injection time is 16min, the detection wavelength is 234nm, the injection volume is 80μL, the collection time is 12.6-13.1min, RT=12.8min, after preparation, all collected solutions are combine...
Embodiment 3
[0049] Implementation 3: Structural identification of Phellodendronine glucuronide conjugates:
[0050] Metabolite M1 is a white amorphous powder, the powder is dissolved in 40% acetonitrile water, and LC-MS n Analytical verification. by LC-MS n The quasi-molecular ion m / z 694[M+H] was obtained by a full-scan mass spectrometry + , which is 352 Da more than the relative molecular mass of the original drug Phellodendriline. Fragment ions m / z 518, m / z 342 and m / z 368 can be obtained in the secondary scanning mass spectrometry, and fragment ions m / z 342, m / z 368 and m / z 192 can be obtained in the tertiary scanning mass spectrometry. Determine m / z 694.23416[M+H] + The molecular formula is C 32 h 40 NO 16 , PDA absorption wavelength is 234nm, 284nm.
[0051] Take 8 mg of metabolite M1 powder and dissolve it in DMSO-d6 at 600M 1 H-NMR spectrum detection and under 150M conditions 13 C-NMR spectrum detection. 1 The high field region in the H-NMR spectrum gives δ3.20 (3H, s, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com