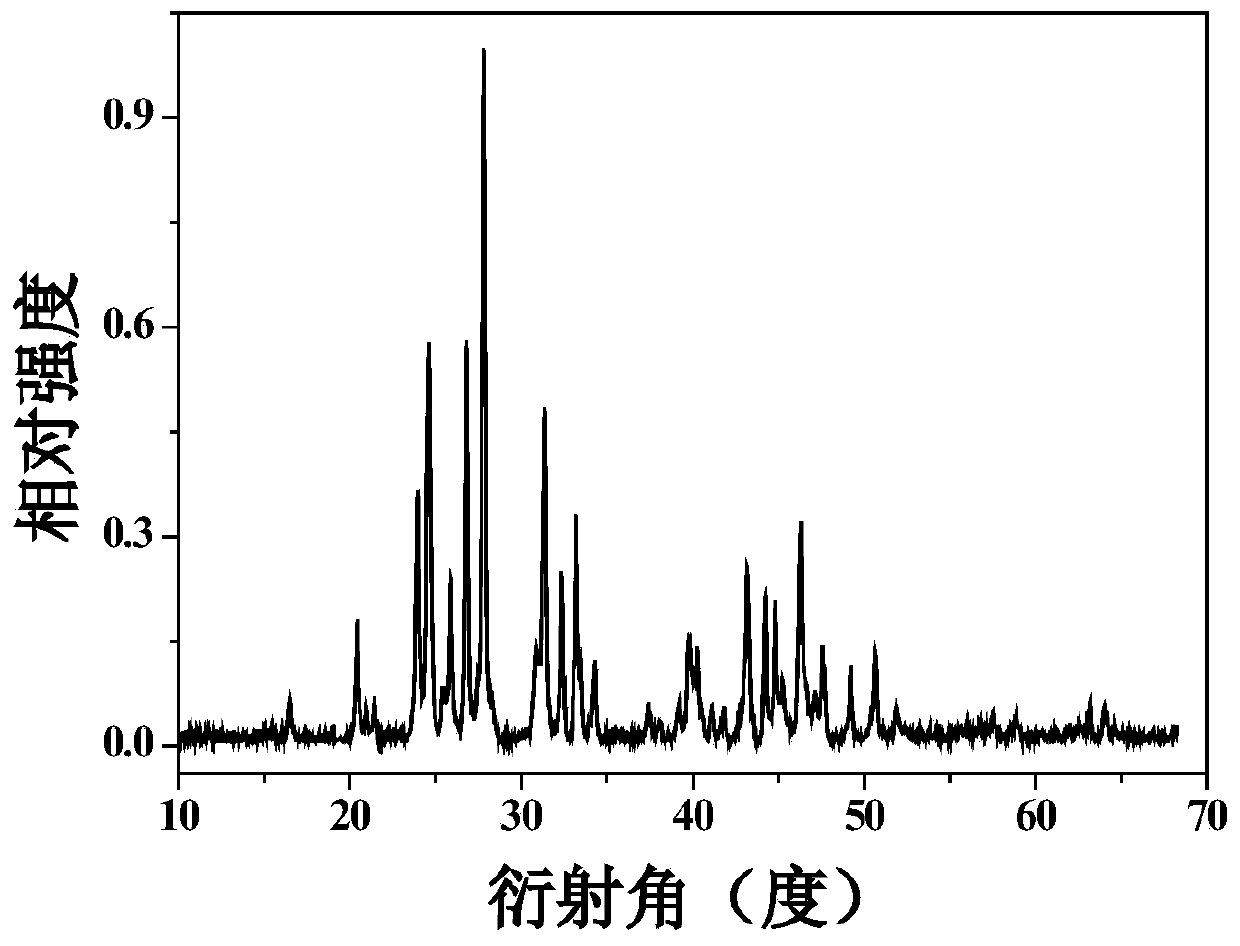
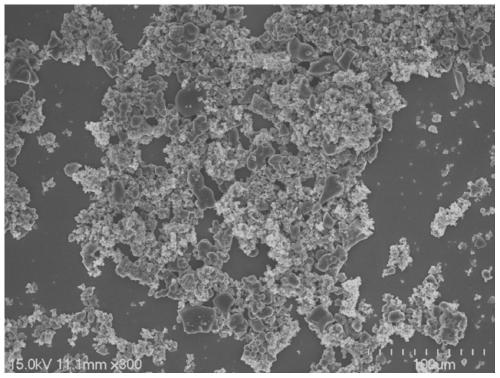
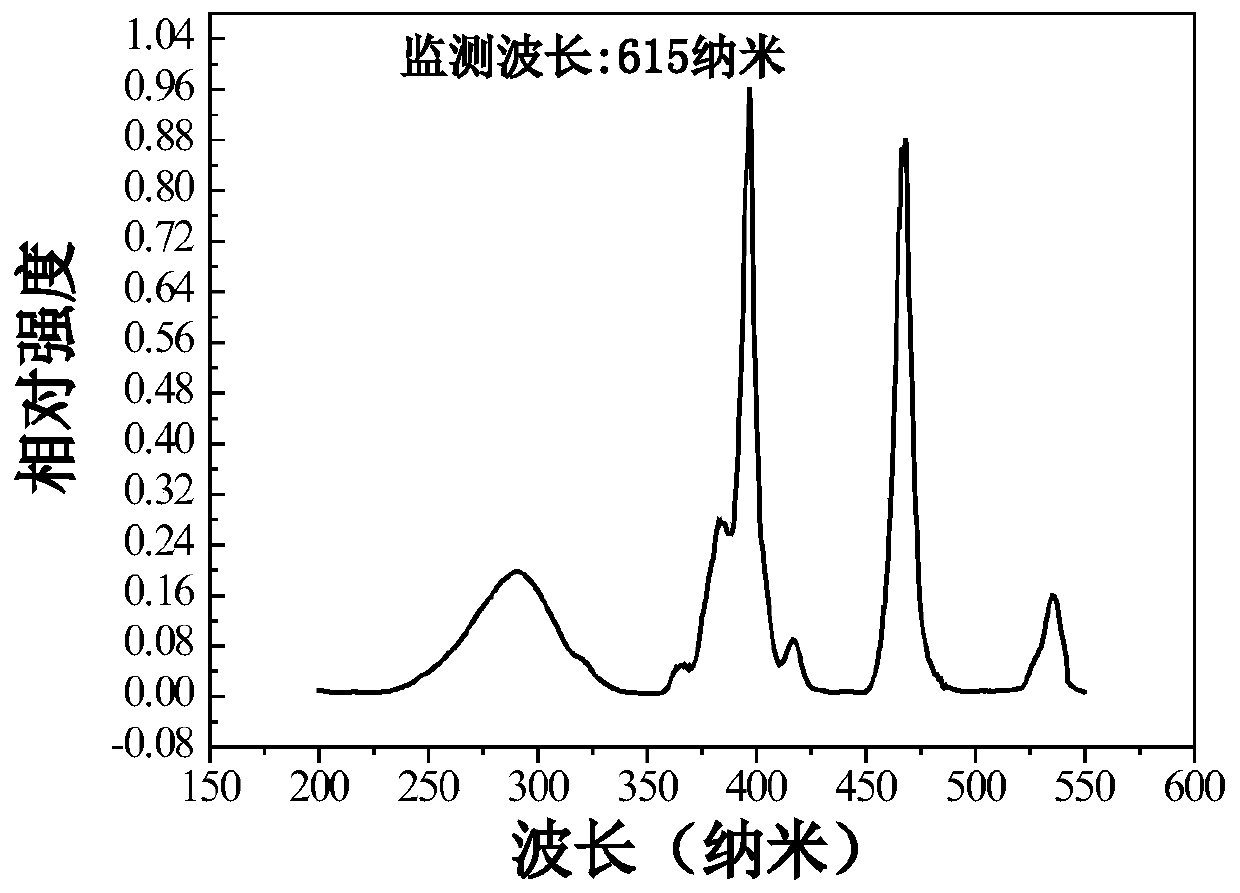
Eu<3+>-activated barium strontium fluoborate red fluorescent powder, preparation and application thereof
A technology of red fluorescent powder and fluoroboric acid, which is applied in the field of inorganic fluorescent materials and displays, can solve the problems of poor chemical stability, corrosion of LED chips, complicated preparation methods, etc., and achieve the effect of uniform particles and strong excitation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Ba 2.75 Eu 0.25 Sr 2 B 4 o 10 f 2
[0040] According to chemical formula Ba 1.75 Eu 0.25 Sr 2 B 4 o 10 f 2 The stoichiometric ratio of Ba, Eu, and Sr elements in the medium is weighed separately: barium carbonate BaCO 3 : 2.76 g, strontium carbonate SrCO 3 : 2.36 grams, europium nitrate Eu (NO 3 ) 3 ·6H 2 O: 0.89 g, dissolved in dilute nitric acid solution to obtain a transparent solution, added 3.52 g of complexing agent oxalic acid, and stirred at 90°C. Then according to the chemical formula Ba 1.75 Eu 0.25 Sr 2 B 4 o 10 f 2 The stoichiometric ratio of the B element in the medium, weigh the boric acid H 3 BO 3 : 1.98 grams, dissolved in dilute nitric acid solution to obtain a transparent solution, adding 3.6 grams of complexing agent oxalic acid, stirring at 90 ° C. The two solutions were slowly mixed, stirred at 100°C for 1 hour and then left to stand, then dried at 200°C to obtain a fluffy powder; the powder was calcined in a muffl...
Embodiment 2
[0047] Example 2: Ba 2.85 Eu 0.15 Sr 2 B 4 o 10 f 2
[0048] According to chemical formula Ba 1.85 Eu 0.15 Sr 2 B 4 o 10 The stoichiometric ratio of Ba, Eu, and Sr elements in the medium is weighed separately: barium carbonate BaCO 3 : 2.19 g, strontium carbonate SrCO 3 : 1.77 grams, europium nitrate Eu (NO 3 ) 3 ·6H 2 O: 0.404 g, dissolved in dilute nitric acid solution to obtain a transparent solution, added 3.21 g of complexing agent oxalic acid, and stirred at 80°C. Then according to the chemical formula Ba 1.85 Eu 0.15 Sr 2 B 4 o 10 The stoichiometric ratio of the B element in the medium, weigh the boric acid H 3 BO 3 : 1.48 grams, dissolved in dilute nitric acid solution to obtain a transparent solution, adding 3.24 grams of complexing agent oxalic acid, stirring at 80 ° C. The two solutions were slowly mixed, stirred at 85°C for 3 hours, left to stand, and then dried at 160°C to obtain a fluffy powder; the powder was calcined in a muffle furnace at...
Embodiment 3
[0051] Example 3: Ba 2.995 Eu 0.005 Sr 2 B 4 o 10 f 2
[0052] According to chemical formula Ba 1.995 Eu 0.005 Sr 2 B 4 o 10 The stoichiometric ratios of Ba, Eu, and Sr elements were weighed respectively: barium oxide BaO: 2.33 grams, strontium oxide SrO: 2.34 grams, europium nitrate Eu (NO 3 ) 3 ·6H 2 O: 0.0014 g, dissolved in dilute nitric acid solution to obtain a transparent solution, added 4.671 g of complexing agent oxalic acid, and stirred at 75°C. Then according to the chemical formula Ba 1.995 Eu 0.005 Sr 2 B 4 o 10 The stoichiometric ratio of the B element in the medium, weigh the boric acid H 3 BO 3: 4.68 grams, dissolved in dilute nitric acid solution to obtain a transparent solution, adding 1.17 grams of complexing agent oxalic acid, stirring at 80 ° C. The two solutions were slowly mixed, stirred at 90°C for 2 hours, left to stand, and then dried at 180°C to obtain a fluffy powder; the powder was calcined in a muffle furnace at a temperature o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com