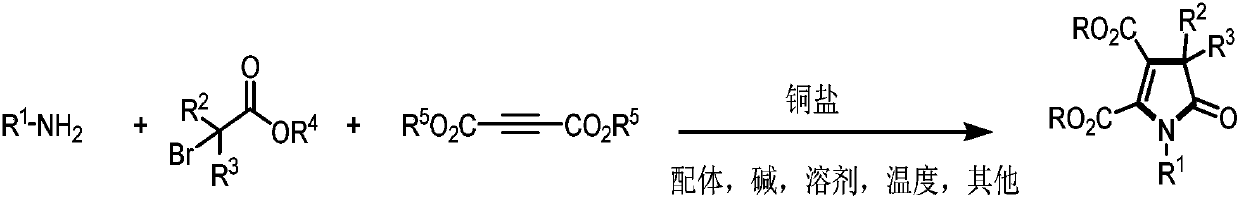
Preparation method of poly-substituted pyrrolidone derivatives
A pyrrolidone and multi-substitution technology, which is applied in the field of preparation of multi-substituted pyrrolidone derivatives, can solve the problems of difficult coupling reaction and limited application, and achieves the effects of high atom utilization, overcoming steric hindrance, and wide substrate range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
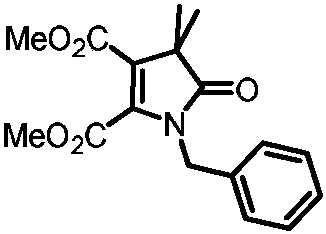
[0031] Preparation of 1-benzyl-4,4-dimethyl-5-oxo-4,5-dihydro-1H-pyrrole-2,3-dicarboxylic acid dimethyl ester
[0032]
[0033] Add 0.1mmol of primary amine compound, 0.2mmol of ethyl 2-bromodimethylpropionate, 0.1mmol of dimethyl butyndioate, and 0.2mol of potassium acetate, add 0.5mL of methyl tert-butyl ether, and place in a nitrogen atmosphere. In a reactor at 120°C, after reacting for 24 hours, cool to room temperature, then filter the solid impurities with diatomaceous earth, wash with dichloromethane three times to obtain the filtrate, then concentrate the filtrate, and then pass through column chromatography or thin film Purified by layer chromatography to obtain 19.7 mg of the target product with a yield of 62%. The nuclear magnetic characterization of this target product is as follows: 1 H NMR (500MHz, Chloroform-d) δ7.31(m, 3H), 7.18-7.11(d, 2H), 4.75(s, 2H), 3.72(s, 3H), 3.64(s, 3H), 1.43( s, 6H); 13 C NMR (126MHz, Chloroform-d) δ181.6, 162.3, 162.2, 142.9, 1...
Embodiment 2
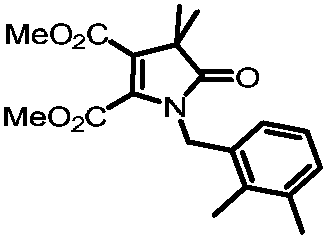
[0035] Preparation of dimethyl 1-(2,3-dimethylbenzyl)-4,4-dimethyl-5-oxo-4,5-dihydro-1H-pyrrole-2,3-dicarboxylate
[0036]
[0037] Add 0.1mmol of primary amine compound, 0.2mmol of ethyl 2-bromodimethylpropionate, 0.1mmol of dimethyl butyndioate, and 0.2mol of potassium acetate, add 0.5mL of methyl tert-butyl ether, and place in a nitrogen atmosphere. In a reactor at 120°C, after reacting for 24 hours, cool to room temperature, then filter the solid impurities with diatomaceous earth, wash with dichloromethane three times to obtain the filtrate, then concentrate the filtrate, and then pass through column chromatography or thin film Purified by layer chromatography to obtain 21.2 mg of the target product with a yield of 62.5%. The nuclear magnetic characterization of this target product is as follows: 1 H NMR (500MHz, Chloroform-d) δ7.09 (d, J=7.4Hz, 1H), 7.04 (t, J=7.6Hz, 1H), 6.86 (dd, J=7.7, 1.3Hz, 1H), 4.80 (s, 2H), 3.71(s, 3H), 3.51(s, 3H), 2.27(s, 3H), 2.11(s, 3H), ...
Embodiment 3
[0039] Dimethyl 1-(3,4-dimethoxybenzyl)-4,4-dimethyl-5-oxo-4,5-dihydro-1H-pyrrole-2,3-dicarboxylate preparation
[0040]
[0041] Add 0.1mmol of primary amine compound, 0.2mmol of ethyl 2-bromodimethylpropionate, 0.1mmol of dimethyl butyndioate, and 0.2mol of potassium acetate, add 0.5mL of methyl tert-butyl ether, and place in a nitrogen atmosphere. In a reactor at 120°C, after reacting for 24 hours, cool to room temperature, then filter the solid impurities with diatomaceous earth, wash with dichloromethane three times to obtain the filtrate, then concentrate the filtrate, and then pass through column chromatography or thin film Purified by layer chromatography to obtain 26.3 mg of the target product with a yield of 70%. The nuclear magnetic characterization of this target product is as follows: 1 H NMR (500MHz, Chloroform-d) δ6.79(d, J=8.4Hz, 1H), δ6.70(d, J=7.3Hz, 2H), δ4.68(s, 2H), δ3.86( s, 3H), δ3.84(s, 3H), δ3.73(s, 3H), δ3.72(s, 3H), δ1.41(s, 6H); 13C NMR (126M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com