Preparation method and application of LaCoO3 nanomaterial with oxygen vacancies
A technology of nanomaterials and oxygen vacancies, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, chemical/physical processes, etc., can solve the problem of non-unique evaluation indicators of geometric area catalysts and low ammonia production rate etc. to achieve good catalytic stability, lower reaction energy barrier, and promote activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] Specifically, the embodiment of the present invention discloses a kind of LaCoO with oxygen vacancies 3 Preparation methods of nanomaterials, including:
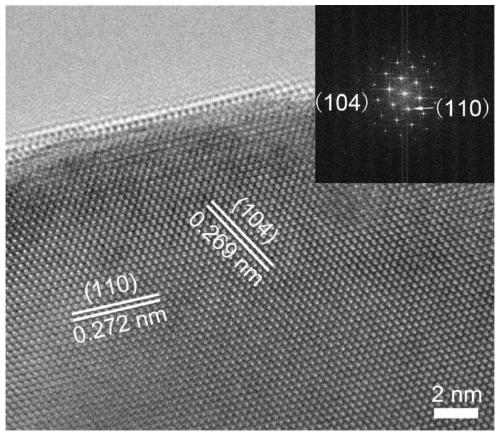
[0038]LaCoO 3 Nanomaterials etched in argon plasma to yield LaCoO with surface oxygen vacancies 3 nanomaterials.
[0039] This application is preparing LaCoO with surface oxygen vacancies 3 In the process of nanomaterials, LaCoO was realized by etching 3 The surface of nanomaterials has oxygen vacancies. LaCoO described in this application 3 Nanomaterials are prepared according to methods well known to those skilled in the art, for example, the LaCoO 3 The preparation method of nanomaterials is:
[0040] Mixing lanthanum source, cobalt source, urea, citric acid monohydrate, water and concentrated nitric acid to obtain a gel;
[0041] The gel is dried after heating, and finally calcined to obtain LaCoO 3 nanomaterials.
[0042] In the above LaCoO 3 In the preparation process of nanomaterials, the lanthanum s...
Embodiment 1
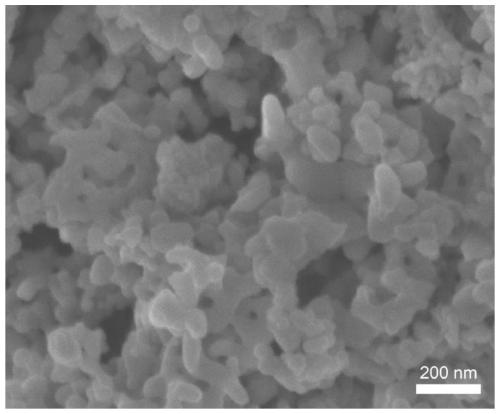
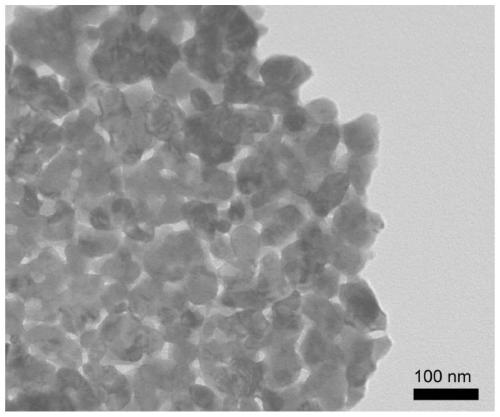
[0054] The present invention provides a LaCoO with surface oxygen vacancies 3 The nano catalyst has an average size of 60 nanometers to 100 nanometers, and its synthesis method is as follows:
[0055] Lanthanum nitrate hexahydrate, cobalt acetate tetrahydrate, urea and citric acid monohydrate were dissolved in 30mL of water, wherein the concentrations of lanthanum nitrate hexahydrate and cobalt acetate tetrahydrate were 0.125 mol / liter, and the concentrations of urea and citric acid monohydrate were both 0.125 mol / liter. 0.5 mol / L, add 3 ml of concentrated nitric acid, mix well, heat to 80°C, and magnetically stir until a gel is formed; then dry at 170°C for 12 hours, and the obtained sample is calcined at 600°C under oxygen for 6 hours to obtain LaCoO 3 nanocatalyst.
[0056] LaCoO 3 The nanocatalyst was placed in argon plasma, the power supply was 200 watts, the argon pressure was maintained at 10 torr, and the zinc oxide nanosheets were etched with argon plasma for 30 min...
Embodiment 2
[0059] 3 mg of LaCoO with surface oxygen vacancies 3 Nano-catalyst, 12 mg of activated carbon and 100 microliters of 5% Nafion solution were dispersed in 2.9 milliliters of ethanol, and ultrasonicated for 1 hour to obtain a uniform solution; then, 5 microliters of the above solution was evenly dropped on a 0.5 cm diameter rotating On the disk electrode, the rotating disk electrode is used as the working electrode, the silver / silver chloride electrode is used as the reference electrode, the graphite rod is used as the counter electrode, and 0.1 mol / liter potassium sulfate solution is used as the electrolyte with 30 milliliters of concentration. It is carried out in an H-type electrolytic cell. The electrolytic cell is separated by a Nafion 115 proton exchange membrane. Before the reaction, it is necessary to pass nitrogen gas for at least 30 minutes to drive away other gases. Nitrogen gas is continuously fed at a rate of 10 ml / min; during the reaction, the anode The generated o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| power | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com