Alpha-diimine nickel metal organic ligand, porous organic polymer and application of porous organic polymer
A metal-organic and nickel diimide technology, which is applied in the direction of nickel-organic compounds, preparation of organic compounds, organic compound/hydride/coordination complex catalysts, etc., can solve the problems of high cost and low catalytic activity of palladium catalysts, etc. Short reaction time, excellent stability, and improved catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0044] A typical embodiment of the present disclosure provides α-diimine nickel metal organic ligand, whose chemical structural formula is:
[0045]
[0046] Another embodiment of the present disclosure provides a method for preparing the above-mentioned organic ligand, which is obtained by ketone-amine condensation reaction of 2,6-diisopropyl-p-iodoaniline and 2,3-butanedione.
[0047] In one or more examples of this embodiment, the ketone-amine condensation reaction condition is: the reaction is carried out at room temperature with formic acid as a catalyst. The room temperature mentioned in the present disclosure refers to the indoor temperature, which is generally 15-30°C.
[0048] The third embodiment of the present disclosure provides monomers for preparing porous organic polymers, the chemical structural formula of which is:
[0049]
[0050] The fourth embodiment of the present disclosure provides a method for preparing the above-mentioned monomer, and anhydrous...
Embodiment 1
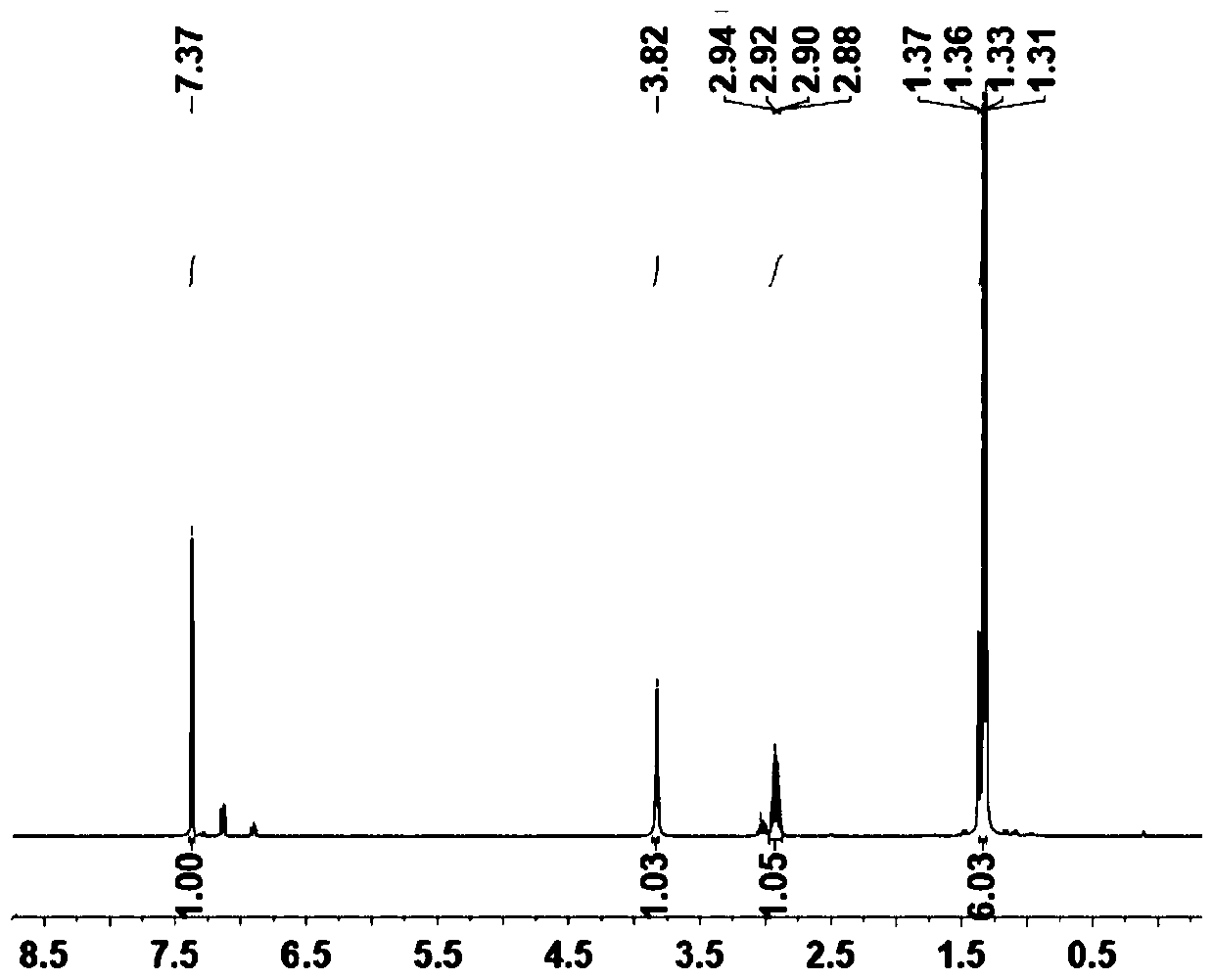
[0070] Example 1: Preparation of α-diimine nickel metal organic ligand (A).
[0071] (1) 2,6-Diisopropylaniline (2.54g, 14.3mmol) and I 2 (4 g, 15.7 mmol) of the mixture was charged to a 50 mL round bottom flask. Then 10 mL of cyclohexane and 4 mL of saturated Na 2 CO 3 solution. After stirring at room temperature for 12 h, diluted with EtOAc (20 mL), saturated Na 2 S 2 o 3 (3 x 40 mL) wash. with anhydrous MgSO 4 The combined organic layer was dried. The crude product was purified by column chromatography (petroleum ether / EtOAc=10 / 1) to obtain 4-iodo-2,6-diisopropylaniline as a black liquid (3.90 g, yield 96%).
[0072] (2) Add 4-iodo-2,6-diisopropylaniline (14mmol, 3.7g) into a 50mL round bottom flask, and then add ditributanedione (7mmol, 0.611mL) into the round bottom flask , add 0.5mL formic acid as catalyst, use 20mL ethanol as solvent, stir at room temperature for 5h, after the reaction, filter with Buchner funnel, wash with a small amount of ethanol, and dry t...
Embodiment 2
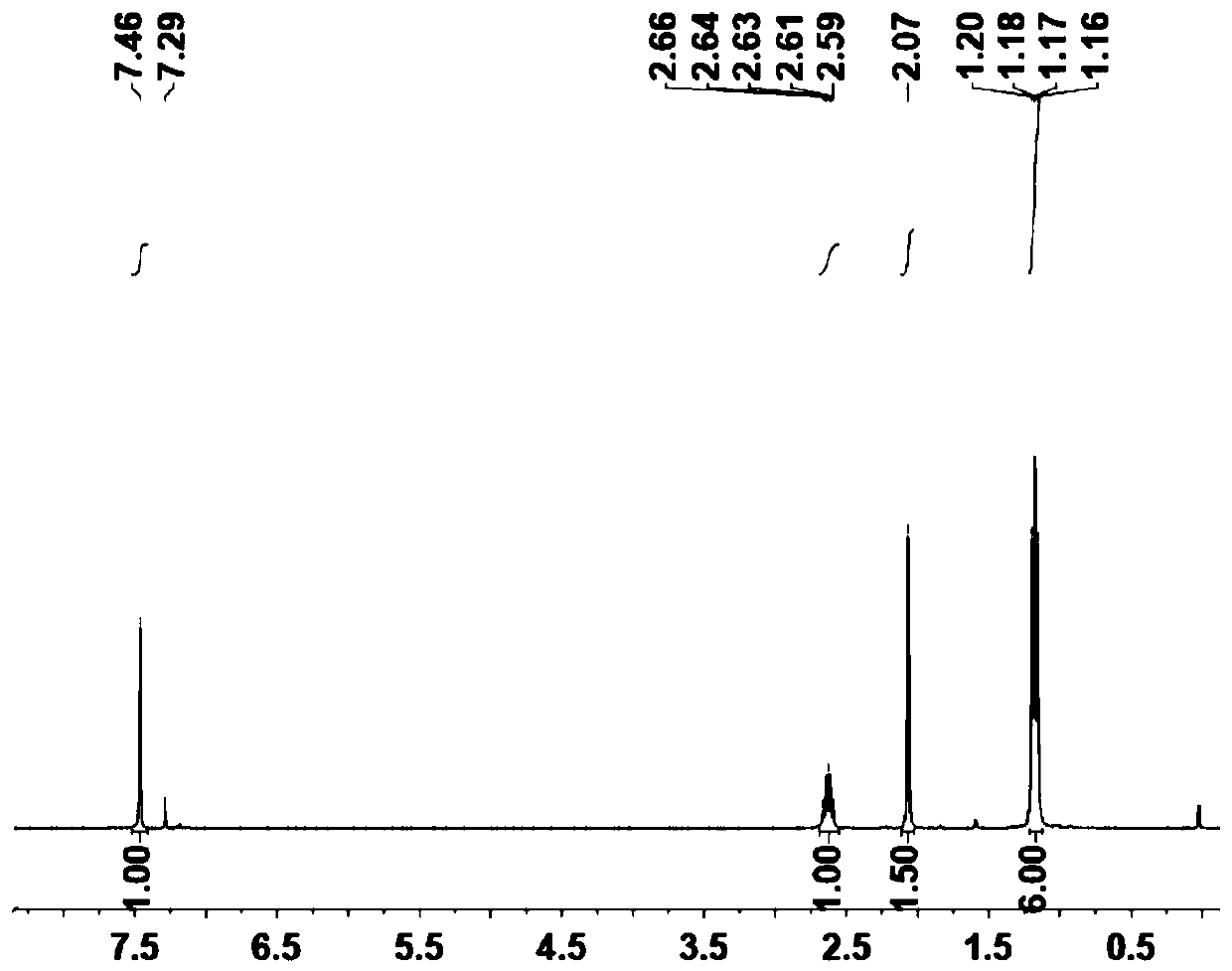
[0080] Example 2: Preparation of monomer (B) of porous organic polymer.
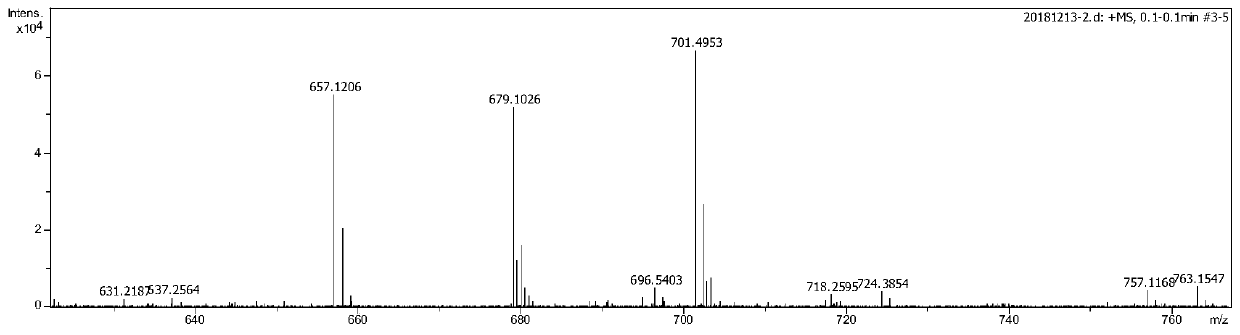
[0081] A (1.1mmol, 0.722g) and DME (NiBr 2 ) (1mmol, 0.308g) was mixed in 50mL of anhydrous dichloromethane and placed in a 100mL Schlenk flask. After stirring at room temperature under nitrogen for 2 days, the reaction was filtered through a pad of celite. The resulting solid was further washed with anhydrous ether and dried in vacuo to obtain B as a brick red solid (0.59g, 68.0%), characterized by the following structure: Figure 3-4 shown.
[0082] The synthetic route of B is as follows:
[0083]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com