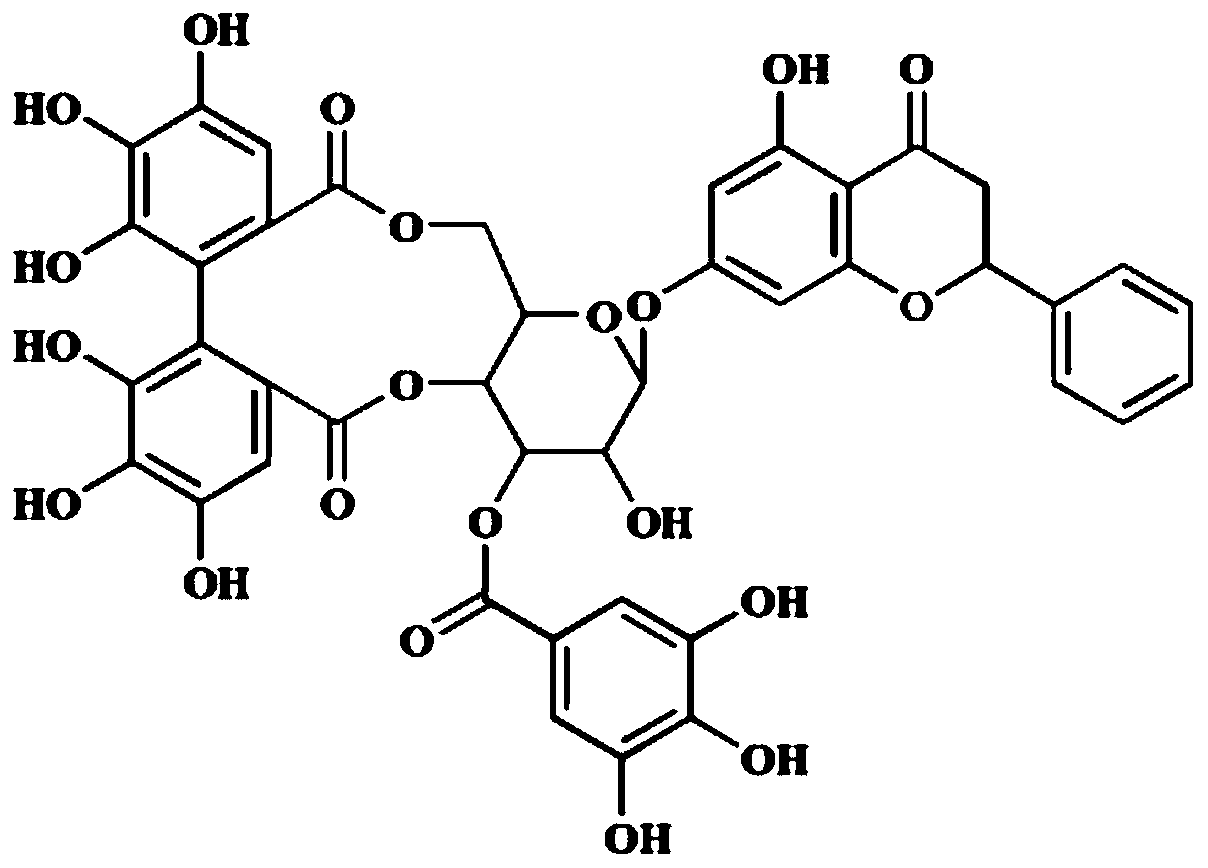
Preparation method of compound PGHG in medicine for treating parkinson's disease
A compound, disease technology, applied in the field of medicine to achieve the effect of improving cell viability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
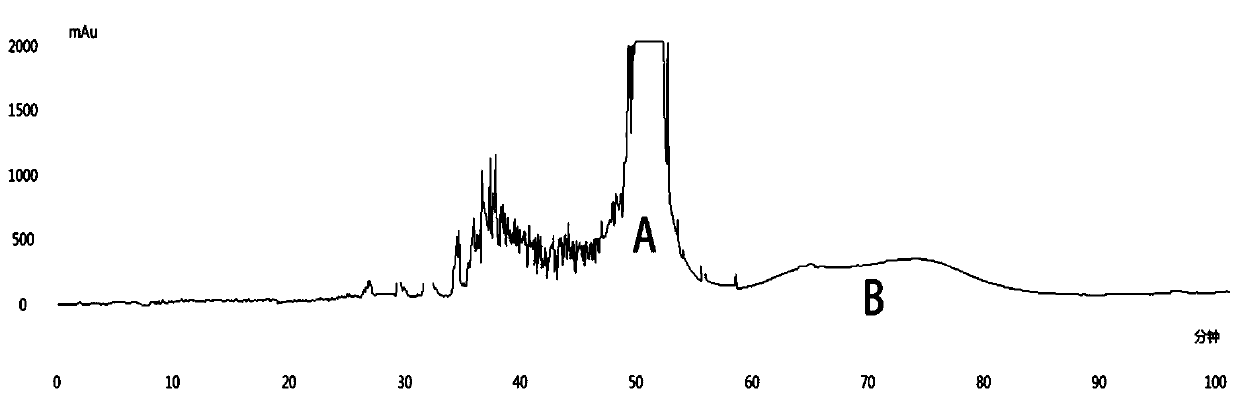
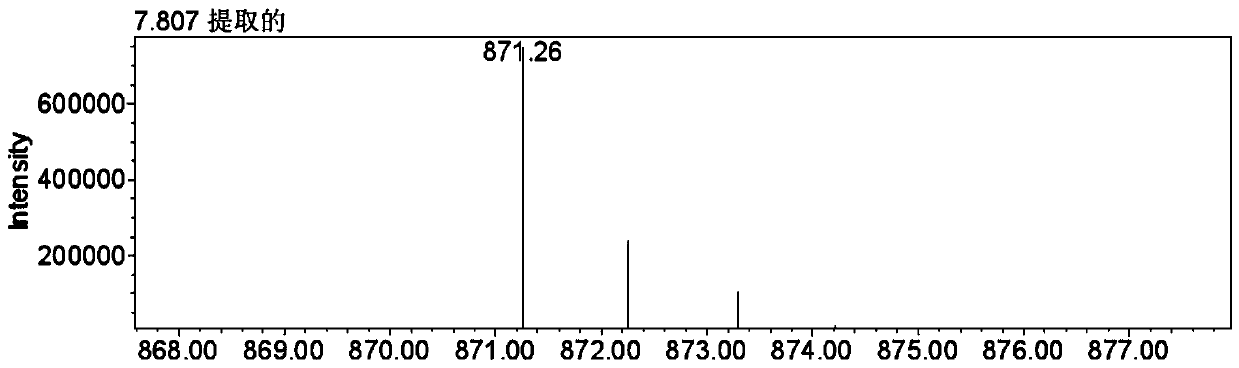
[0020] Embodiment 1: the separation method of PGHG
[0021] 1.1 Establishment of separation method
[0022] Use 25 times the volume of 70% ethanol (solid-to-liquid ratio = 1:25) to extract the dry powder of the yellow grass stem ultrasonically at 55°C and 59kHz, repeat twice, 40min each time, and the filtrates obtained by suction filtration are combined and rotated Concentrate under reduced pressure with an evaporator, then add 200mL ultrapure water to resuspend, extract with ethyl acetate (10 times) according to the volume ratio of 1:1, concentrate under reduced pressure and freeze-dry to obtain the polyphenol fragments (PSE) .
[0023] Use n-hexane-ethyl acetate-methanol-water (2:5:2:5, v / v / v / v) solvent system, the upper phase is used as the mobile phase to elute, the flow rate is 5.0mL / min, the speed is 800rpm, and the positive and negative Switch mode, the detection wavelength is 280nm, the injection volume is 200mg (200mg sample is dissolved in 20mL upper phase), and th...
Embodiment 2
[0030] Example 2: Intervention effect of polyphenol fragments and PGHG on 6-hydroxydopamine (6-OHDA)-induced Parkinson's disease in SH-SY5Y cells
[0031] 2.1 Cell Viability Detection
[0032]The SH-SY5Y cells were inoculated into 96-well plates, and 12 hours later, the control group and the model group were added to the medium, and the drug group was added with 1, 2, 5, 10 μg / mL polyphenol fragments (PSE), 1, 2, and 5 , 10μM GHG and 20μM baicalein (Bai) were treated for 12h, then the model group and the drug group were treated with 200μM 6-OHDA for 12h, and the control group was added with fresh medium. Finally, the liquid in each well was aspirated, and 100 μL of medium (containing 10 μL CCK-8) was added to incubate in an incubator. After 40 min, the absorbance value was measured at 450 nm with a microplate reader, and the cell survival rate was calculated according to the OD value: OD drug / OD control.
[0033] 2.2 ATP level detection
[0034] The SH-SY5Y cells were inocul...
Embodiment 3
[0037] Example 3: Effect of PGHG on 6-OHDA-induced SH-SY5Y cell anti-oxidation, apoptosis and inflammatory protein expression
[0038] Inoculate SH-SY5Y cells into a 60mm culture dish. After the confluence of the cells reaches 70-80%, add medium to the control group and model group, add 2, 4, and 8μMPGHG to the drug group for 12 hours, and then add the model group and drug group 200μM 6-OHDA was treated for 12 hours, and fresh medium was added to the control group. Aspirate the culture medium, digest with trypsin, collect the cells into a 1.5mL centrifuge tube, centrifuge at 2000rpm, 4°C for 7min, aspirate the supernatant, add 200μL RIPA lysate to the cell pellet, lyse by vortexing for 15s, and lyse 3 times at intervals 5min, finally 15000rpm, centrifuge at 4°C for 20min, collect the supernatant, measure the protein concentration with the BCA protein concentration assay kit, add 5xloadingbuffer according to the ratio of 4:1 after leveling, boil, SDS-PAGE protein electrophoresi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com