A kind of nickel-cobalt-manganese ternary positive electrode material and its preparation method and application
A positive electrode material, nickel-cobalt-manganese technology, applied in the field of nickel-cobalt-manganese ternary positive electrode material and its preparation, can solve problems such as side reactions, achieve the effects of reducing material impedance, high cycle retention rate, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
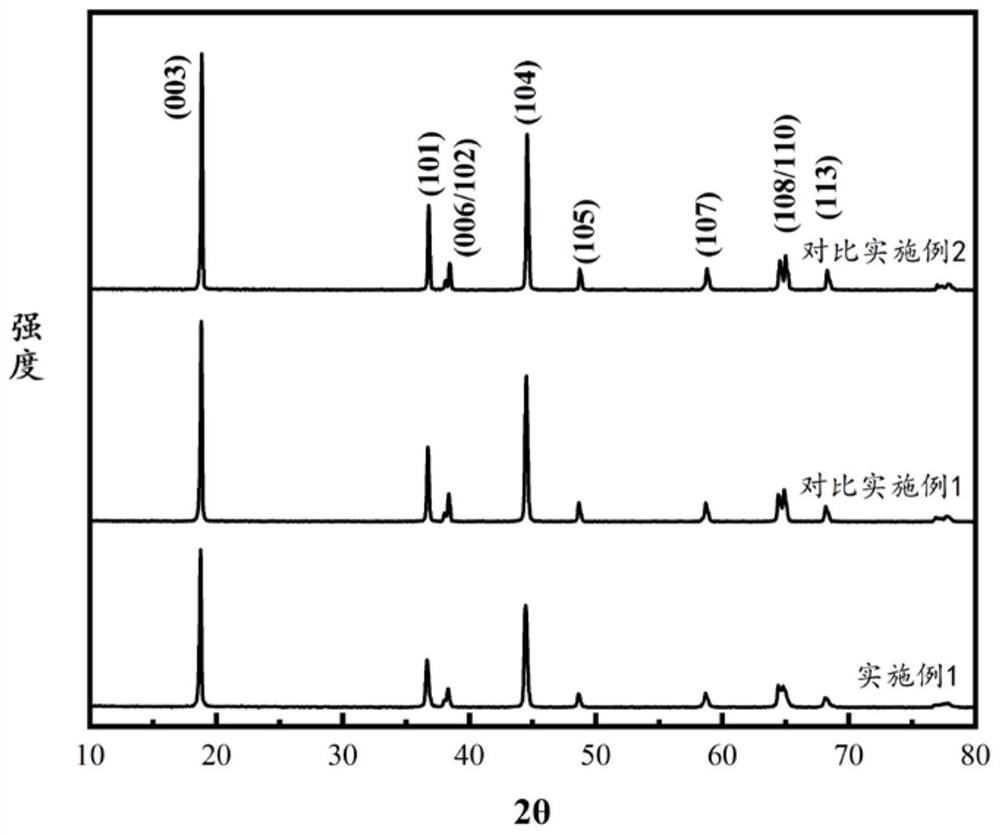
Image
Examples
Embodiment 1
[0036] The ternary precursor molecular formula of the present invention is Ni 0.8 co 0.1 mn 0.1 (OH) 2 , with a D50 of 10.54 μm and an average pore size of 4 nm.
[0037] (1) Weigh 10.00g of the precursor, pour it into a beaker containing 50ml of absolute ethanol solution, and stir and mix at room temperature for 0.5h.
[0038] (2) Weigh 0.053g of H 3 BO 3 (accounting for 0.8mol% of precursor) is dissolved in the pure water of 10ml, stirs to H 3 BO 3 completely dissolved.
[0039] (3) Pour the solution in step (2) into the solution in step (1), stir at room temperature for 10 h, and evaporate to dryness in a water bath at 90° C. to obtain a solid powder.
[0040] (4) Measure the solid powder in step (3), calculate the molar amount of transition metal in the powder, and carry out lithium compounding according to the transition metal to lithium molar ratio of 1:1.05. After the solid powder and lithium salt are evenly mixed, in a pure oxygen atmosphere, the flow rate is ...
Embodiment 2
[0059] The ternary precursor of the present invention chooses molecular formula to be Ni 0.8 co 0.1 mn 0.1 (OH) 2 The precursor of D50 is 10.54μm, and the average pore diameter is 4nm.
[0060] (1) Weigh 10.00g of the precursor, pour it into a beaker containing 50ml of absolute ethanol solution, and stir and mix at room temperature for 0.5h.
[0061] (2) Weigh 0.040g of H 3 BO 3 (accounting for 0.6mol% of precursor) is dissolved in the pure water of 10ml, stirs to H 3 BO 3 completely dissolved.
[0062] (3) Pour the solution in step (2) into the solution in step (1), stir evenly at room temperature for 10 h, stir and evaporate the obtained solution in a water bath at 90° C. to obtain a solid powder.
[0063] (4) Weigh the solid powder in step (3), calculate the molar amount of the precursor metal in the powder, and carry out lithium compounding according to the ratio of the molar amount of metal to the molar ratio of lithium salt as 1:1.05. After mixing the solid powd...
Embodiment 3
[0066] The ternary precursor of the present invention chooses molecular formula to be Ni 0.8 co 0.1 mn 0.1 (OH) 2 The precursor of D50 is 10.54μm, and the average pore diameter is 4nm.
[0067] (1) Weigh 10.00g of the precursor, pour it into a beaker containing 50ml of anhydrous n-propanol solution, and stir and mix at room temperature for 0.5h.
[0068] (2) Weigh 0.053g of H 3 BO 3 Dissolve in 10ml of pure water, stir until H 3 BO 3 completely dissolved.
[0069] (3) Pour the solution in step (2) into the solution in step (1), stir evenly at room temperature for 10 h, stir and evaporate the obtained solution in a water bath at 120° C. to obtain a solid powder.
[0070] (4) Weigh the solid powder in step (3), calculate the molar amount of the precursor metal in the powder, and carry out lithium compounding according to the ratio of the molar amount of metal to the molar ratio of lithium salt as 1:1.05. After mixing the solid powder and lithium salt evenly, in a pure o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com