Slow release dry suspension agent containing azoxystrobin and tebuconazole and preparation method of slow release dry suspension agent
A technology of dry suspension containing azoxystrobin, which is applied in the field of slow-release dry suspension and its preparation, and can solve the problem of no cross-resistance of fungicides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
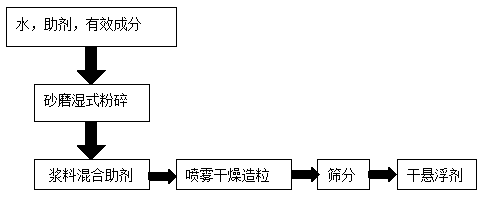
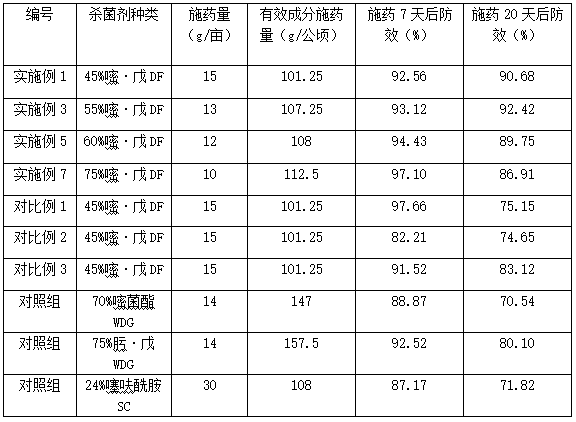
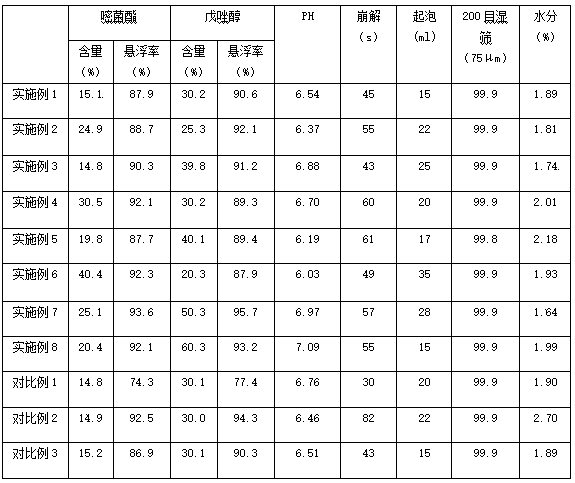
Image
Examples
Embodiment 1
[0030] Example 1 45% azoxystrobin tebuconazole dry suspension
[0031] Azoxystrobin 15%, Tebuconazole 30%, Lincomycin 0.2%, Wetting agent sodium alkyl sulfate 2%, Binder amylose corn starch 3%, Dispersant calcium lignosulfonate 10%, Polymer Carboxylic acid sodium salt 10%, defoamer 1%, grinding aid magnesium stearate 1%, disintegrant urea 10%, filler is sodium sulfate to make up to 100%, pre-add water in the feeding kettle (liquid and solid ratio The ratio is 1:1), the materials are put into the feeding kettle in order, sheared to a homogeneous phase, and sanded by a sand mill until the particle size d98≤5μm enters the finished kettle. Add 2% polyvinyl alcohol stabilizer, 2% cyclodextrin, 0.3% xanthan gum, and 0.5% carboxymethyl cellulose into the finished product kettle, and continue to stir evenly. After the material is filtered, it is transported to the top of the tower for atomization through a high-pressure pump. The inlet air temperature was set at 120°C, the outlet ai...
Embodiment 2
[0032] Example 2 50% azoxystrobin tebuconazole dry suspension
[0033] Azoxystrobin 25%, tebuconazole 25%, lincomycin 0.1%, wetting agent sodium alkylsulfonate 1.5%, binder amylose corn starch 3%, dispersant agent sodium lignosulfonate 12%, Polycarboxylate sodium salt 8%, defoamer 1%, grinding aid magnesium stearate 1%, disintegrant ammonium sulfate 10%, filler is sodium sulfate to make up to 100%, pre-add water (liquid and The solid ratio is 1:1), the materials are put into the feeding kettle in order, sheared to a homogeneous phase, and sanded by a sand mill until the particle size d98≤5μm enters the finished kettle. Add 2% sodium carboxymethyl starch stabilizer, 1% cyclodextrin, 0.3% xanthan gum, and 1.5% modified chitosan to the finished product kettle, and continue to stir evenly. After the material is filtered, it is transported to the top of the tower for atomization through a high-pressure pump. The inlet air temperature was set at 118°C, the outlet air temperature w...
Embodiment 3
[0034] Example 3 55% azoxystrobin tebuconazole dry suspension
[0035] Azoxystrobin 15%, Tebuconazole 40%, Lincomycin 0.3%, Wetting agent Sodium Isopropyl Naphthalene Sulfonate 1.5%, Binder Amylose Corn Starch 3%, Dispersant Sodium Lignosulfonate 13% %, polycarboxylate sodium salt 10%, defoamer 1%, grinding aid magnesium stearate 1%, disintegrant urea 5%, filler is sodium sulfate to make up to 100%, pre-add water in the feeding kettle (liquid The ratio of solid to solid is 1:1), and the materials are put into the feeding kettle in order to cut to a homogeneous phase, and then sanded by a sand mill until the particle size d98≤5μm enters the finished kettle. Add 4% of stabilizer polyvinyl alcohol, 3% of cyclodextrin, 0.2% of xanthan gum, and 2% of modified chitosan into the finished product kettle, and continue to stir evenly. After the material is filtered, it is transported to the top of the tower for atomization through a high-pressure pump. The inlet air temperature was se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com