A potassium-air battery comprising a polyaniline-carbon nanotube-tin dioxide-polyacrylonitrile composite nanofiber separator
A composite nanofiber, air battery technology, applied in fuel cells, nanotechnology, nanotechnology and other directions, can solve the problems of potassium-air battery discharge termination, prone to chemical reactions, anode corrosion failure and other problems, achieve excellent electrochemical activity, promote The effect of uniform migration and improved battery performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
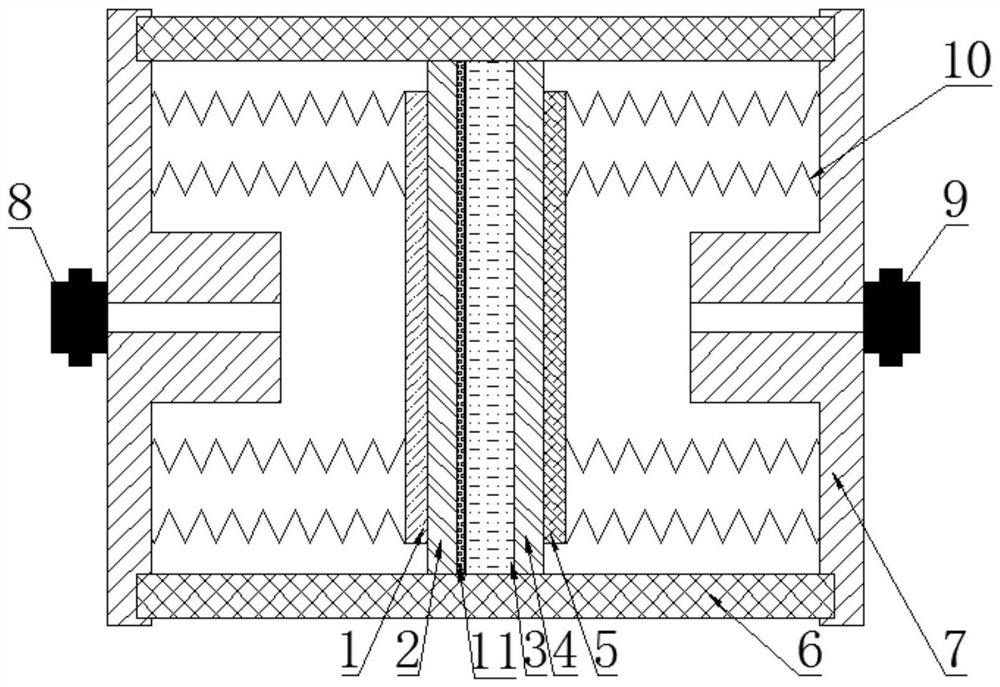
[0025] As shown in the figure, the polyaniline-carbon nanotube-tin oxide-polyacrylonitrile (PCSP) composite separator potassium-air battery includes a stainless steel casing 7 and insulating cover plates 6 arranged on the top and bottom of the stainless steel casing 7. The middle part of the insulating cover plate 6 in the stainless steel shell 7 is sequentially provided with a potassium battery negative electrode 1, a first microporous polyolefin diaphragm 2, an electrolyte 3, a second microporous polyolefin diaphragm 4, a potassium battery positive electrode 5, and the potassium air An argon gas chamber is left between the negative electrode 1 of the battery and the stainless steel shell 7 close to the negative electrode 1 of the potassium air battery, and an argon gas valve 8 is provided on the stainless steel shell 7 on one side of the argon gas chamber. An air chamber is left between the positive electrode 5 and the stainless steel shell 7 near the positive electrode 5 of ...
Embodiment 2
[0039]As shown in the figure, the structure of the polyaniline-carbon nanotube-tin oxide-polyacrylonitrile (PCSP) composite separator potassium air battery is the same as that of Example 1.
[0040] Preparation of PCSP Composite Separator
[0041] (1) Preparation of spinning solution
[0042] 0.6g of polyacrylonitrile (PAN) was added to the mixed solvent of 1g of chloroform and 6.5g of N,N-dimethylformamide (DMF), and stirred at room temperature for 4h to obtain a uniform colloidal liquid; 0.1800g of aniline and 0.216g of Camphorsulfonic acid was added to the colloidal solution and stirred at room temperature for 4 hours, then 0.45 g of ammonium persulfate was added and stirred at room temperature for 60 minutes, the solution was placed in a refrigerator at 5 °C for 48 hours, and then 0.1 g of carbon nanotubes ( CNT) and 0.1g SnO 2 Dissolved in the above solution and stirred for 24h to obtain a spinning solution;
[0043] (2) Preparation of PCSP separator
[0044] A 1mL pl...
Embodiment 3
[0050] As shown in the figure, the structure of the polyaniline-carbon nanotube-tin oxide-polyacrylonitrile (PCSP) composite separator potassium air battery is the same as that of Example 1.
[0051] Preparation of PCSP Composite Separator
[0052] (1) Preparation of spinning solution
[0053] 0.6g of polyacrylonitrile (PAN) was added to the mixed solvent of 1g of chloroform and 6.5g of N,N-dimethylformamide (DMF), and stirred at room temperature for 2h to obtain a uniform colloidal liquid; 0.1800g of aniline and 0.216 g camphorsulfonic acid was added to the colloidal solution and stirred at room temperature for 2 hours, then 0.45 g of ammonium persulfate was added and stirred at room temperature for 30 minutes, the solution was placed in a refrigerator at 5°C for 24 hours, and then 0.1 g of carbon nanotubes were added (CNT) and 0.1g SnO 2 Dissolved in the above solution and stirred for 12h to obtain a spinning solution;
[0054] (2) Preparation of PCSP separator
[0055] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com