Doxycycline hydrochloride tablet, preparation method thereof, application and antibacterial drug
A doxycycline hydrochloride tablet technology, applied in the field of medicine, can solve the problems of poor safety and effectiveness of finished tablets, high loss rate of dry granulation, poor quality stability, etc., and achieve good fluidity and compressibility , Improve safety and effectiveness, high uniformity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
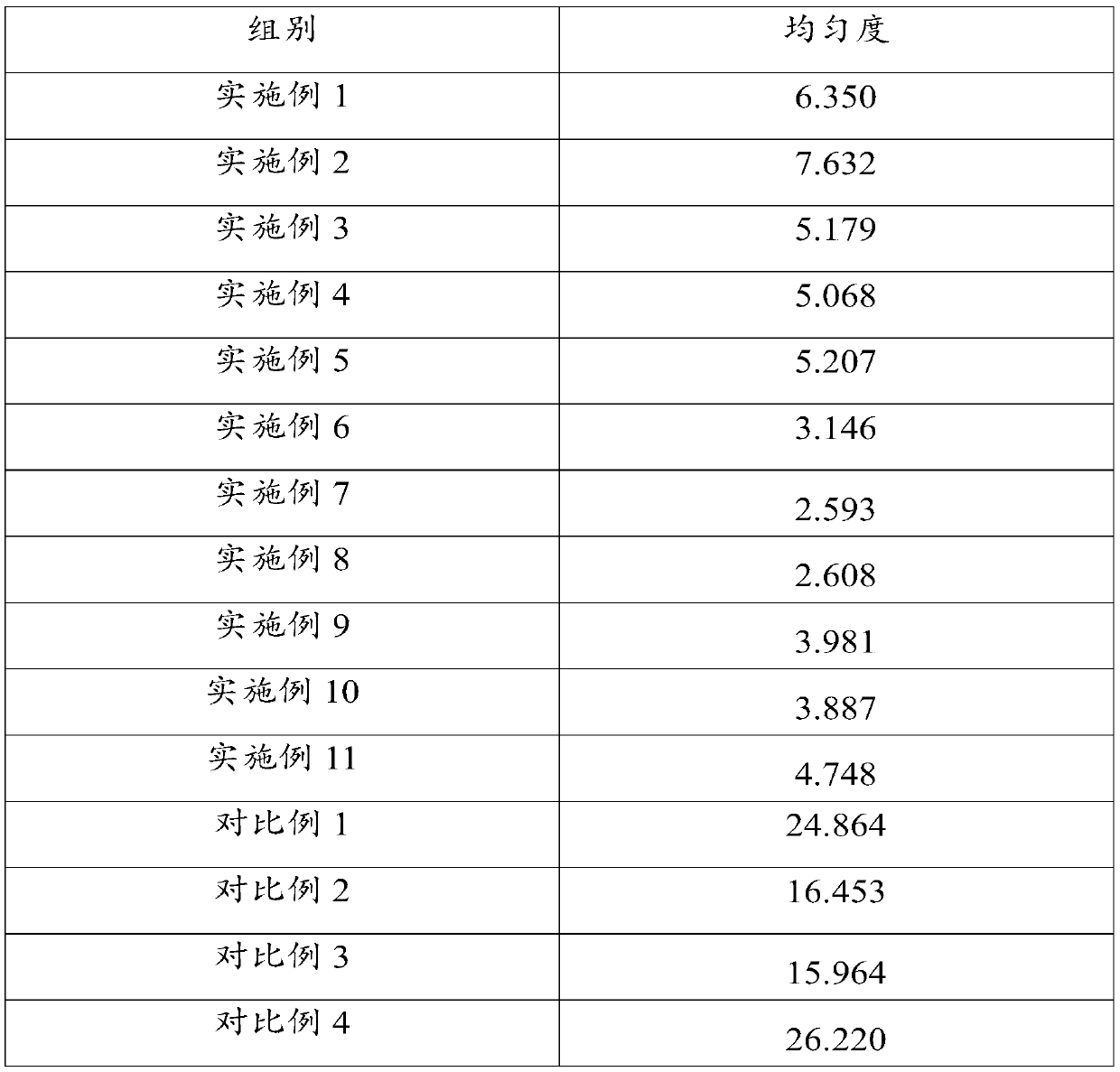
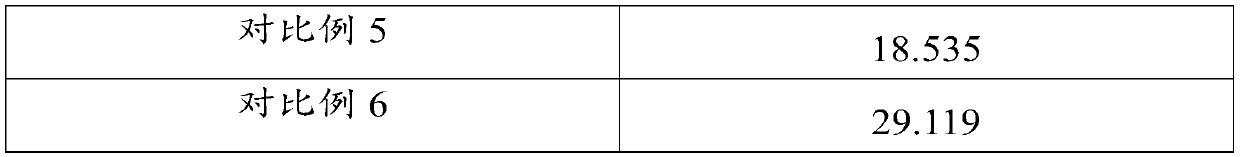
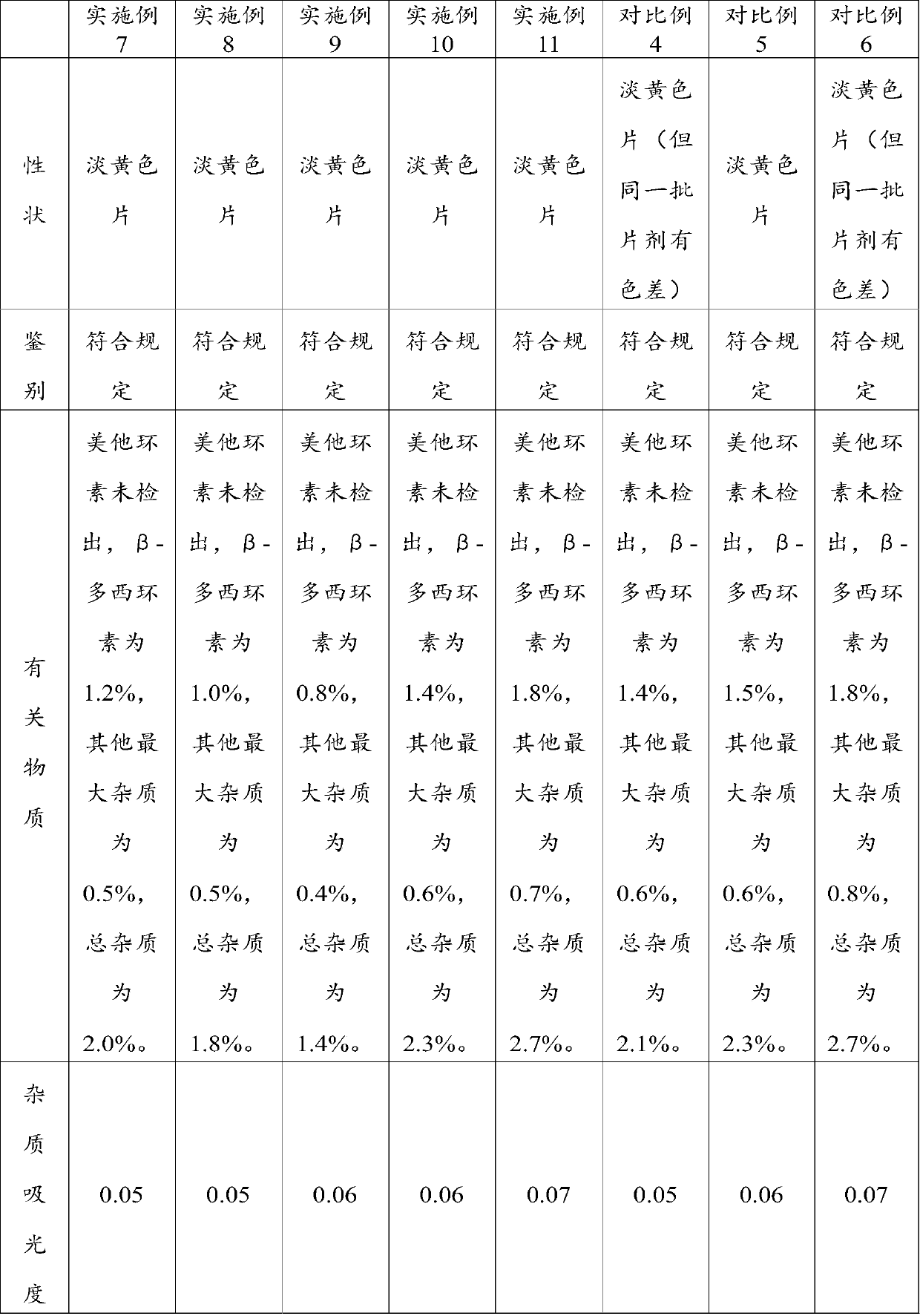
[0075] According to another aspect of the present invention, a method for preparing the above-mentioned doxycycline hydrochloride tablet is provided, comprising: mixing all raw materials uniformly and then compressing the tablet to obtain the doxycycline hydrochloride tablet. The method has a simple process and can be directly pressed into tablets after the raw materials are evenly mixed without using wet or dry granulation procedures, avoiding high temperature and high humidity conditions, ensuring the stability of product quality, and improving the safety and effectiveness of tablets. The tablet has the advantages of high uniformity, high hardness and smooth surface. In addition, the method also has low requirements on production equipment, and can effectively save the consumption of various raw materials and the cost of manpower and equipment loss.
[0076] In a preferred embodiment, the mixing includes: first premixing the doxycycline hydrochloride, the glidant and the fir...
Embodiment 1
[0098] A doxycycline hydrochloride tablet, mainly prepared from the following raw materials in mass percentage: 10% doxycycline hydrochloride, 2% silicon dioxide, 0.5% magnesium stearate and 87.5% silicified microcrystalline cellulose %.
Embodiment 2
[0100] A doxycycline hydrochloride tablet, mainly prepared from the following raw materials in mass percentage: 30% of doxycycline hydrochloride, 5% of silicon dioxide, 4% of magnesium stearate and 61% of silicified microcrystalline cellulose %.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com