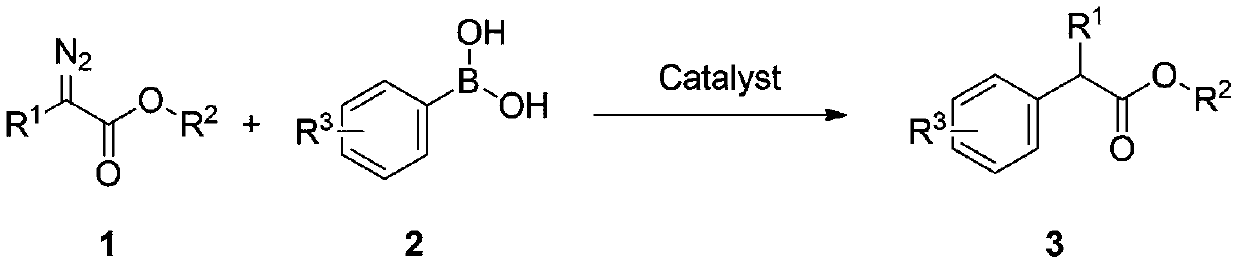
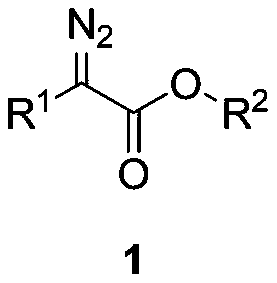
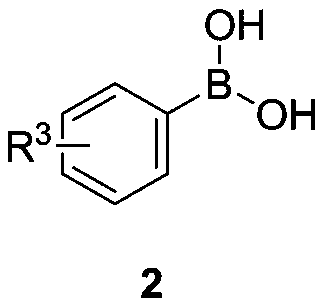
Method for synthesizing aryl acetate derivative under catalysis of surface modified sludge charcoal
A technology for aryl acetate and surface modification, which is applied in the preparation of organic compounds, chemical instruments and methods, and preparation of carboxylate esters. It can solve the problems that have not been reported in relevant literature, and achieve easy separation and purification and simple preparation. , to achieve the effect of reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] Preparation of sludge charcoal: domestic sewage sludge from Dalian sewage treatment plant, China, dried in a muffle furnace at 80-200°C to constant weight (optimum temperature 105°C), and heated at a flow rate of 200-1000mL / min Heating to 400-800°C (optimum temperature 600°C) under pure nitrogen (optimum flow rate 500mL / min) and heating rate 1-10°C / min (optimum heating rate 3°C / min), continuous carbonization 2 -8h (the best carbonization time is 4h), when the temperature in the furnace drops to room temperature, sludge carbon (SW) can be obtained.
[0040] Preparation of surface modified sludge charcoal: soak sludge charcoal in the modifier for 24 hours, wash with deionized water until the pH of the washing solution reaches 3-7, then recover the solid and dry it at room temperature to obtain Surface modified sludge charcoal.
[0041] Wherein, the modifying agent is HClO 4 (concentration: 10-70wt%, optimum concentration: 35.4wt%), HCl (concentration: 10-50wt%, optimum ...
Embodiment 1
[0044]
[0045] At room temperature, successively weigh ethyl phenyldiazoacetate 1a (0.5mmol), phenylboronic acid 2a (0.8mmol), SW-I (50mg) in a 25mL Schlenk reaction tube, and add 1, 5mL of 2-dichloroethane, stirred at room temperature for 0.5h, moved to a 70°C oil bath with magnetic stirring heater, and reacted for 6 hours. TLC detected that the raw materials had reacted completely, stopped the reaction, cooled the reaction solution to room temperature, and filtered it with suction , the filter cake was washed with dichloromethane until the filtrate was colorless, concentrated under reduced pressure to remove volatile components, and separated by silica gel column chromatography (eluent: petroleum ether (60-90°C) / ethyl acetate, v / v=10: 1), the target product 3a (61 mg, yield 51%) was obtained in a colorless, transparent and viscous form, and the target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 2
[0046] Embodiment 2 (comparative example: different catalyst contrasts)
[0047]
[0048] At room temperature, successively weigh ethyl phenyldiazoacetate 1a (0.5mmol), phenylboronic acid 2a (0.8mmol), SW-Ⅴ (50mg) in a 25mL Schlenk reaction tube, add 1, 5mL of 2-dichloroethane, stirred at room temperature for 0.5h, moved to an oil bath magnetic stirring heater at 70°C to react for 6 hours, TLC detected that the raw materials were not completely reacted (contrast experiment, ensure the same reaction time), stop the reaction, Cool the reaction solution to room temperature, filter with suction, wash the filter cake with dichloromethane until the filtrate is colorless, concentrate under reduced pressure to remove volatile components, and separate by silica gel column chromatography (eluent: petroleum ether (60-90°C) / acetic acid Ethyl ester, v / v=10:1), the target product 3a (37 mg, yield 31%) was obtained as colorless, transparent and viscous, and the target product was confirme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com