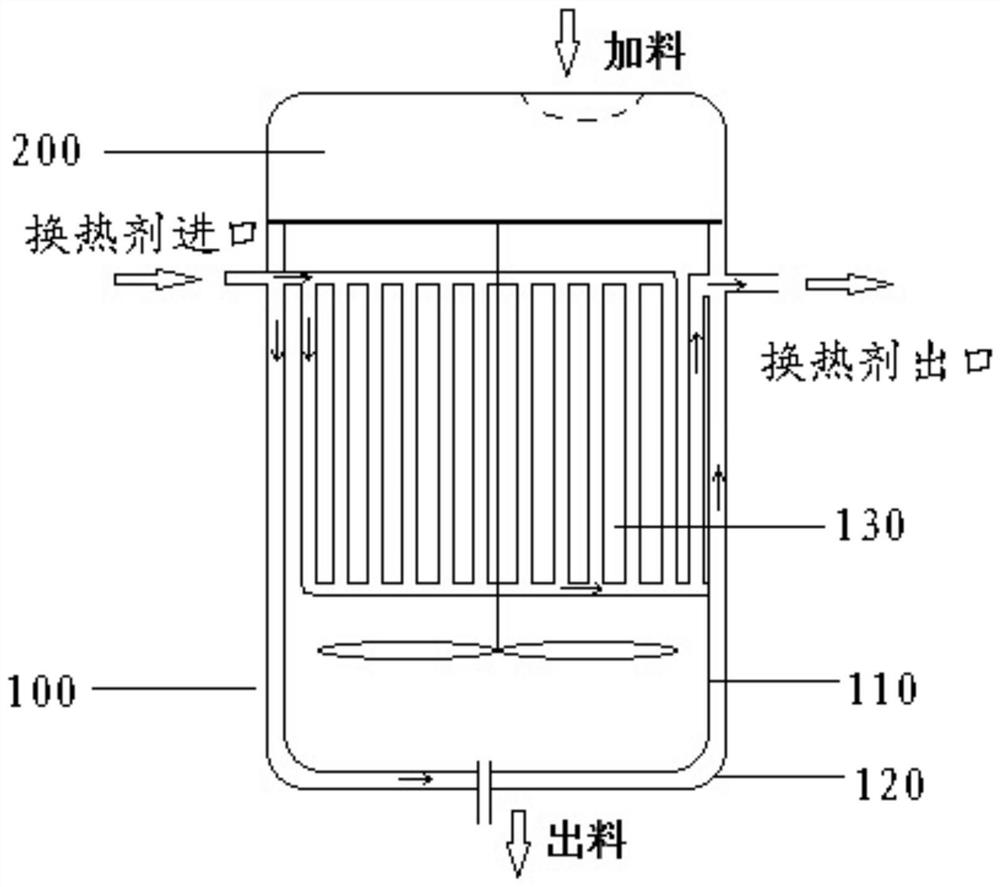
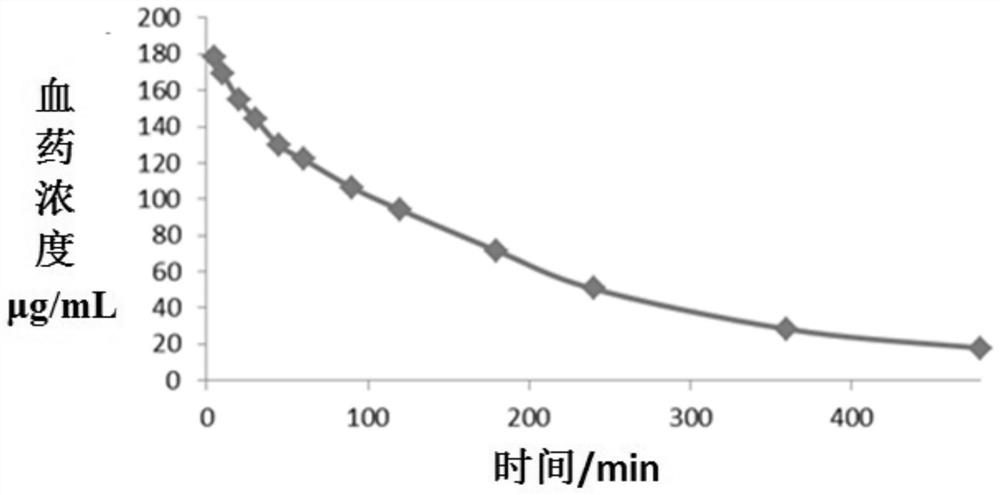
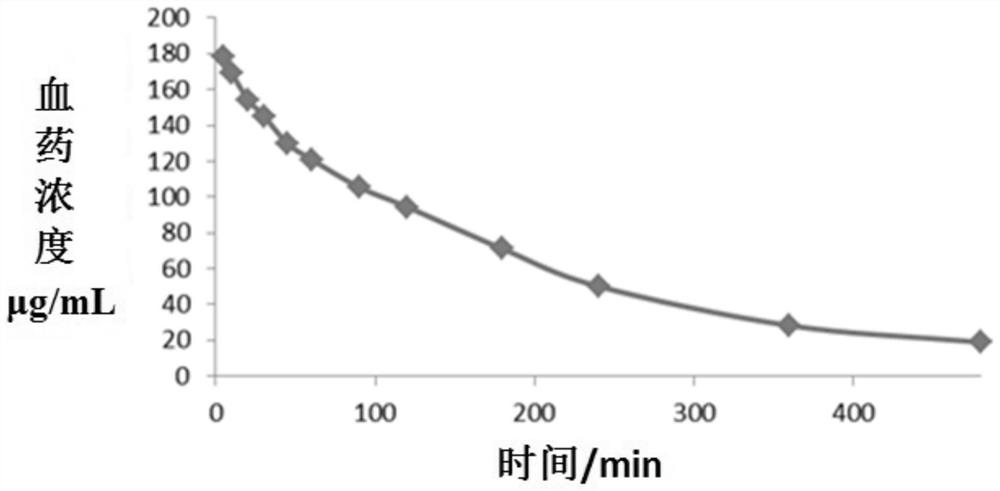
Cefoperazone sodium sulbactam sodium composition pharmaceutical preparation and new indications for treating infective endocarditis
The technology of cefoperazone sodium and sulbactam sodium is applied in the new indication field of cefoperazone sodium and sulbactam sodium pharmaceutical preparation for the treatment of infective endocarditis, which can solve the problem of weakening the stability of the finished preparation, light exposure of cefoperazone sodium, strong acid, strong base, oxidation It is beneficial to long-term storage and placement, reducing the risk of clinical sensitization, and improving the safety of use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0143] The synthesis of embodiment 1-1 cefoperazone sodium
[0144] Step 1), dissolve 136g (0.5mol) 7-ACA, 40g (0.55mol) MTT in 420mL dimethyl carbonate, cool down to 5°C, add AlCl 3 -BF 3 Dimethyl carbonate composite catalyst 4.6g (wherein, AlCl 3 0.67g (0.005mol), BF 3 1.7g (0.025mol)), warmed up to 30°C, and reacted for 3h under stirring. After the reaction, cool the reaction liquid to 10°C, add 400mL water and 2g EDTA-Na 2 , add 126g (1.5mol) of sodium bisulfate in batches within 20min, stir for 1h, keep the temperature below 5°C, pH 2.0, crystallization occurs, continue to stir for 1h, filter with suction, wash with acetone, acetic acid water, and acetone respectively, and dry to obtain White solid powder compound I (7-ACT) 161.5g, yield 98.4%, HPLC content greater than 98%.
[0145] Step 2), dissolve 167.7g (0.5mol) of piperazine acid in 500mL N,N-dimethylformamide (DMF), cool the reaction solution to -5°C, add 51.9 g (0.175mol) of The toluene solution of bis(tric...
Embodiment 1-2
[0148] The synthesis of embodiment 1-2 cefoperazone sodium
[0149] Identical to the synthesis process of Example 1-1, the only difference is: in step 1), in step 1), AlCl 3 -BF 3 AlCl in dimethyl carbonate composite catalyst 3 and BF 3 The weight ratio is 1:8.
Embodiment 2-1
[0150] The synthesis of embodiment 2-1 sulbactam sodium
[0151] Step A), cool 275mL (3.0mol) of 25% hydrobromic acid to 0°C, add dropwise dilute sulfuric acid to adjust the pH to 2.0; weigh 120mL water and 108g 6-APA (0.5mol) to prepare a suspension, cool to 0°C, add dropwise to the hydrobromic acid solution; weigh 34.5g (0.5mol) of sodium nitrite and dissolve it in 50mL of water, add dropwise to the above system, stir continuously during the dropwise addition, and continue to stir the reaction after the dropwise addition is completed 2h, the temperature was controlled at 0-5°C during the reaction. After the reaction is completed, 14 (weight)% hydrogen peroxide (1.5 mol) is added dropwise to the above system, and the reaction is stirred for 1 hour after the dropwise addition, and the temperature is controlled at 0-5° C. during the reaction. After the reaction was completed, 15% sodium bisulfite was slowly added until the solution turned colorless, and the end point was detec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com