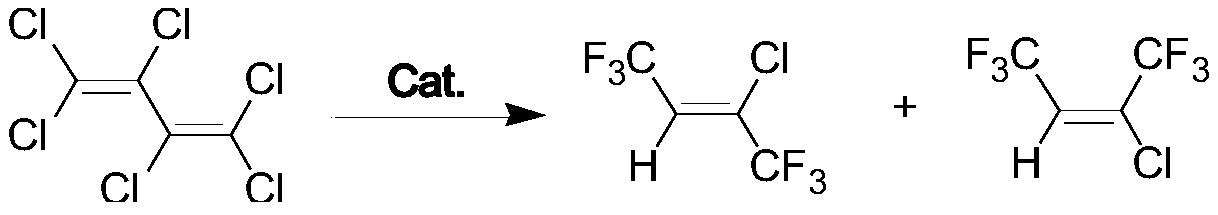
Conversion method for hexachlorobutadiene
A technology for hexachlorobutadiene and butene, applied in the field of catalytic conversion of hexachlorobutadiene, can solve the problems of inability to recycle, poor thermal stability, low reactivity, etc., and achieves high industrial application prospects and thermal stability. High, low reactivity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 45 grams of alum pentoxide, 50 grams of anhydrous hydrogen fluoride, 20 grams of potassium fluoride and 10 grams of anhydrous sulfuric acid into a 300mL stainless steel belt stirred autoclave. Vacuum to remove excess water.
[0020] Add 52 grams of hexachlorobutadiene and 40 grams of anhydrous hydrogen fluoride into the kettle, then raise the temperature to 150°C for 3-4 hours, control the pressure at 1.5-1.7 MPa, and collect the discharged material with a cold trap.
[0021] The product is detected by NMR, and the data are as follows:
[0022] 1 H NMR (500MHz, CDCl 3 )δ6.64(q,1H,J=6.5Hz);
[0023] 13 C NMR (500MHz, CDCl 3 )δ132.0(qq, J=39.3Hz, J=5.4Hz), 121.9(q, J=37.5Hz), 120.6(q, J=270Hz), 119.1(q, J=272.5Hz);
[0024] 19 F NMR (470MHz, CDCl 3 )δ-71.3(m, CF 3 ),-61.3(m, CF 3 ).
[0025] The above data prove that the product obtained is 2-chloro-1,1,1,4,4,4-hexafluoro-2-butene.
Embodiment 2~8
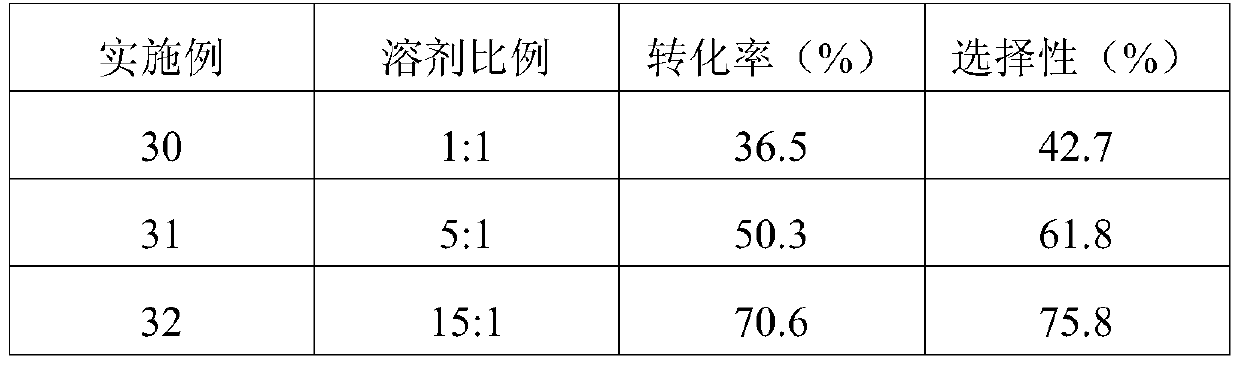
[0027] Examples 2-8 Prepare 2-chloro-1,1,1,4,4,4-hexafluoro-2-butene according to the same preparation method in Example 1, the difference is that the catalyst in Example 1 is Alum pentoxide, and the ratio of telomerization in Examples 2 to 8 is titanium dioxide, niobium pentoxide, tantalum pentoxide, titanium tetrachloride, alum pentachloride, niobium pentachloride, and tantalum pentachloride. The reaction results of Examples 2-8 are shown in Table 1.
[0028] Table 1 Catalyst Screening
[0029] Example catalyst Conversion rates(%) selectivity (%) 2 TiO 2
Embodiment 9~13
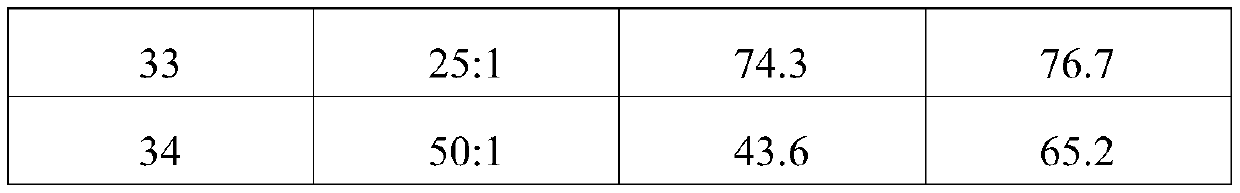
[0031] Examples 9 to 13 were prepared according to the same preparation method in Example 1, 2-chloro-1,1,1,4,4,4-hexafluoro-2-butene, the difference is that the catalyst Agent is potassium fluoride, and in embodiment 9~13, be respectively tripropylamine, tributylphosphine, potassium bisulfate, potassium chlorosulfonate, potassium benzenesulfonate. The reaction results of Examples 9-13 are shown in Table 2.
[0032] Table 2 Screening of catalytic promoters
[0033] Example Catalyst Conversion rates(%) selectivity (%) 9 Tripropylamine 65.1 81.1 10 Tributylphosphine 61.3 72.3 11 Potassium bisulfate 78.2 80.1 12 Potassium chlorosulfonate 80.3 83.0 13 Potassium benzenesulfonate 78.5 73.4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com