Polymer electrolyte membrane and solid-state battery
An electrolyte membrane and polymer technology, used in non-aqueous electrolyte batteries, electrolyte battery manufacturing, electrolytes, etc., can solve problems such as high price, no significant increase in electrical conductivity, and difficulty in improving compatibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] After diluting sodium dodecylbenzene sulfonate with water, first perform proton exchange with hydrochloric acid, and then add lithium hydroxide to prepare a neutral solution to obtain lithium dodecyl benzene sulfonate with a solid content of about 10%.
[0053] Mix PEO with a molecular weight of 400,000 g / mol and lithium dodecylbenzenesulfonate according to a mass ratio of 9:1, add deionized water, seal, heat and stir at 55°C for 24 hours to disperse uniformly to obtain a composite slurry.
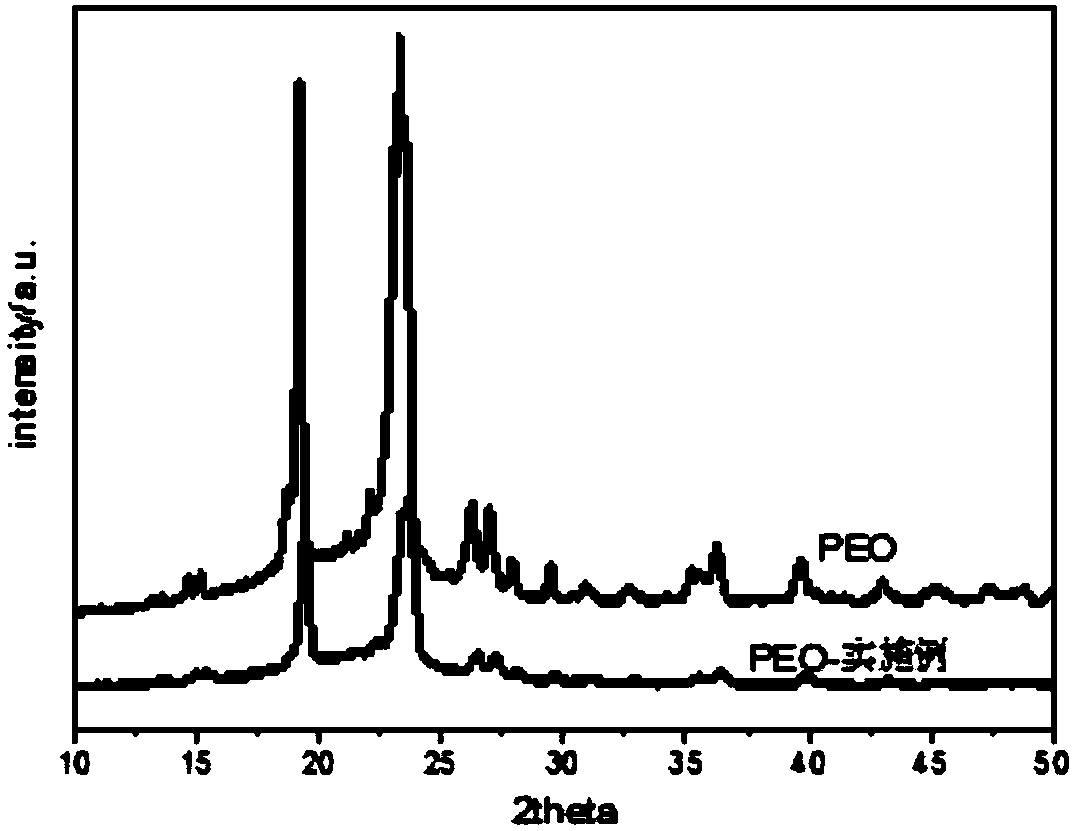
[0054] Using the solution casting method, the composite slurry was placed in a polytetrafluoroethylene mold and dried at room temperature for 48 hours. The 14mm original sheet was dried in a vacuum oven at 55°C for 24h to obtain a polymer electrolyte membrane.
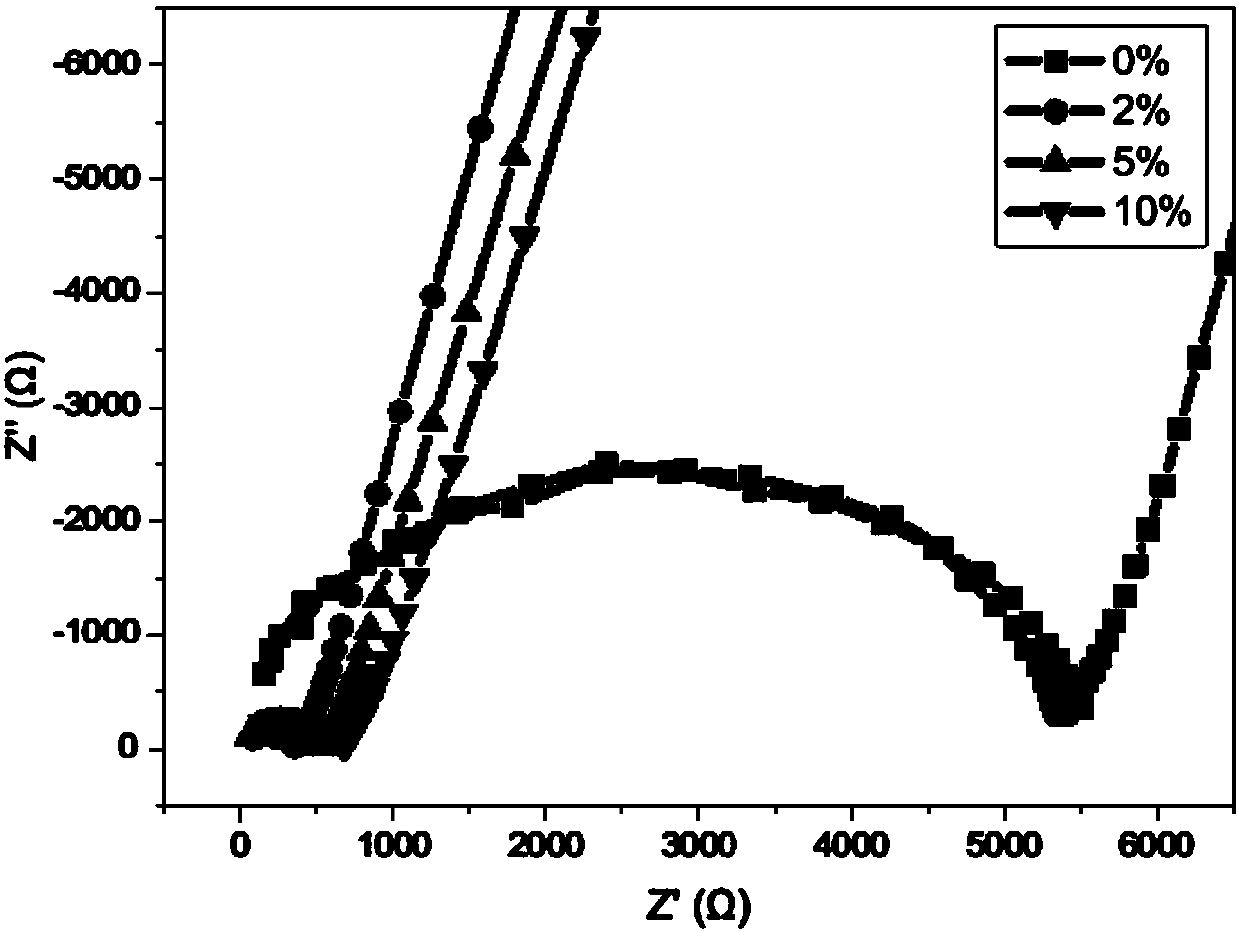
[0055] The CR2032 solid-state battery was assembled, with stainless steel electrodes on both sides, and the conductivity was tested. The results are shown in Table 1.
Embodiment 2
[0057] After diluting sodium dodecylbenzene sulfonate with water, first perform proton exchange with hydrochloric acid, and then add lithium hydroxide to prepare a neutral solution to obtain lithium dodecyl benzene sulfonate with a solid content of about 10%.
[0058] Mix PEO with a molecular weight of 200,000 g / mol and lithium dodecylbenzenesulfonate in a mass ratio of 49:1, add deionized water, seal, heat and stir at 55°C for 24 hours to disperse evenly; add LiTFSI lithium salt, and the oxygen-to-lithium ratio is 15: 1, to obtain a composite slurry.
[0059] Using the solution casting method, the composite slurry was placed in a polytetrafluoroethylene mold and dried at room temperature for 48 hours. The 14mm original sheet was dried in a vacuum oven at 55°C for 24h to obtain a polymer electrolyte membrane.
[0060] The CR2032 solid-state battery was assembled, with stainless steel electrodes on both sides, and the conductivity was tested. The results are shown in Table 1....
Embodiment 3
[0068] After diluting sodium dodecylbenzene sulfonate with water, first perform proton exchange with hydrochloric acid, and then add lithium hydroxide to prepare a neutral solution to obtain lithium dodecyl benzene sulfonate with a solid content of about 10%.
[0069] Mix PEO with a molecular weight of 300,000 g / mol and lithium dodecyl benzene sulfonate at a mass ratio of 19:1, add deionized water, seal, heat and stir at 55°C for 24 hours to disperse evenly; add LiTFSI lithium salt, and the oxygen-to-lithium ratio is 15: 1, to obtain a composite slurry.
[0070] Using the solution casting method, the composite slurry was placed in a polytetrafluoroethylene mold. After drying at room temperature for 48 hours, it was shot into an original sheet of φ14mm and dried in a vacuum oven at 55°C for 24 hours to obtain a polymer electrolyte membrane.
[0071] The CR2032 solid-state battery was assembled, with stainless steel electrodes on both sides, and the conductivity was tested. The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com