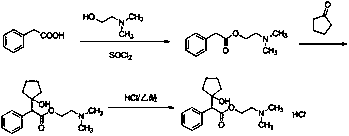
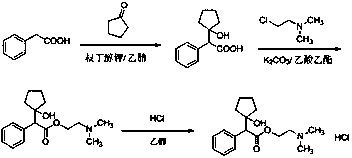
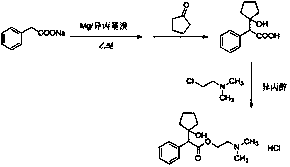
Preparation method of cyclopentolate hydrochloride intermediate
A technology for cyclopentolate and intermediates, which is applied in the field of preparation of cyclopentatropate hydrochloride intermediates, can solve the problems of high labor protection requirements for workers, high humidity requirements, unfavorable safety production and the like, and achieves reduction of production risks and a The requirements of labor protection for workers, the reduction of crystallization and impurity removal procedures, and the effect of environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Preparation of Phenylacetic Acid
[0041] Add 300ml of purified water and 8.4g (0.21mol) of sodium hydroxide to the reaction flask, stir, dissolve, add 30.0g (0.20mol) of methyl phenylacetate, heat up to 50°C, stir for 2h, cool down to T=10°C , 18.4ml (0.23mol) of hydrochloric acid was added dropwise, stirred for 1h, filtered, and dried to obtain 25.9g of phenylacetic acid (95.1% yield), with a purity of 99.9% (HPLC area purity method).
[0042] 2. Preparation of 2-(1-hydroxycyclopentyl)-phenylacetic acid
[0043] Add 300ml of 1M phenylmagnesium chloride tetrahydrofuran solvent into the reaction flask, raise the temperature to 40°C, add dropwise 50ml of tetrahydrofuran containing 17.7g (0.13mol) of phenylacetic acid, after the dropwise completion, keep stirring for 2h, then add 12.0g (0.14mol) of cyclopentanone dropwise. mol), the temperature was lowered to 0°C for 2 hours after dropping, and the reaction liquid was quenched by adding 500 ml of 5% hydrochloric acid ...
Embodiment 2
[0045] 1. Preparation of Phenylacetic Acid
[0046] Add 300ml of purified water and 15.2g (0.11mol) of potassium carbonate to the reaction flask, stir, dissolve, add 30.0g (0.20mol) of methyl phenylacetate, raise the temperature to 60°C, stir for 3h, cool down to T=0°C, 18.4ml (0.23mol) of hydrochloric acid was added dropwise, stirred for 2h, filtered, and dried to obtain 25.9g of phenylacetic acid (93.6% yield) with a purity of 99.8% (HPLC area purity method).
[0047] 2. Preparation of 2-(1-hydroxycyclopentyl)-phenylacetic acid
[0048] Add 300ml of 1M isopropylmagnesium chloride tetrahydrofuran solvent into the reaction flask, heat up to 30°C, add dropwise 50ml of tetrahydrofuran containing 17.7g (0.13mol) of phenylacetic acid, after the dropwise completion, keep stirring for 1h, then add dropwise 12.0g of cyclopentanone ( 0.14mol), after dropping, keep it warm for 1 hour, cool down to 0°C, quench the reaction solution by adding 500ml of 10% hydrochloric acid aqueous solut...
Embodiment 3
[0050] The phenylacetic acid obtained in Example 1 was used as a raw material to prepare 2-(1-hydroxycyclopentyl)-phenylacetic acid.
[0051] Add 150ml of 2M m-toluene magnesium chloride tetrahydrofuran solvent into the reaction flask, add 50ml of tetrahydrofuran containing 17.7g (0.13mol) of phenylacetic acid dropwise at room temperature, after the dropwise completion, stir for 2h, add dropwise 12.0g (0.14mol) of cyclopentanone, dropwise After that, the temperature was raised to 35°C for 1 hour, and the temperature was lowered to 0°C. The reaction solution was quenched by adding 300ml of 15% aqueous hydrochloric acid solution prepared in advance, and extracted by adding 100ml of ethyl acetate. Evaporate to dryness under reduced pressure to obtain an oil, add cyclohexane to beat at room temperature for 8 hours, filter, and dry to obtain 24.5 g of 2-(1-hydroxycyclopentyl)-phenylacetic acid (yield 85.6%) with a purity of 95.2% (HPLC area normalization one method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com