Oral supplement fluid salt powder and preparation method thereof
A technology for rehydration salts and prescriptions, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, active ingredients of alkaline/alkaline earth metal chlorides, etc., can solve the problems of unfavorable children taking, no clear improvement, and high sodium and glucose content. , to achieve the effect of reducing the necessity, reducing the osmotic pressure, and reducing the amount of feces
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
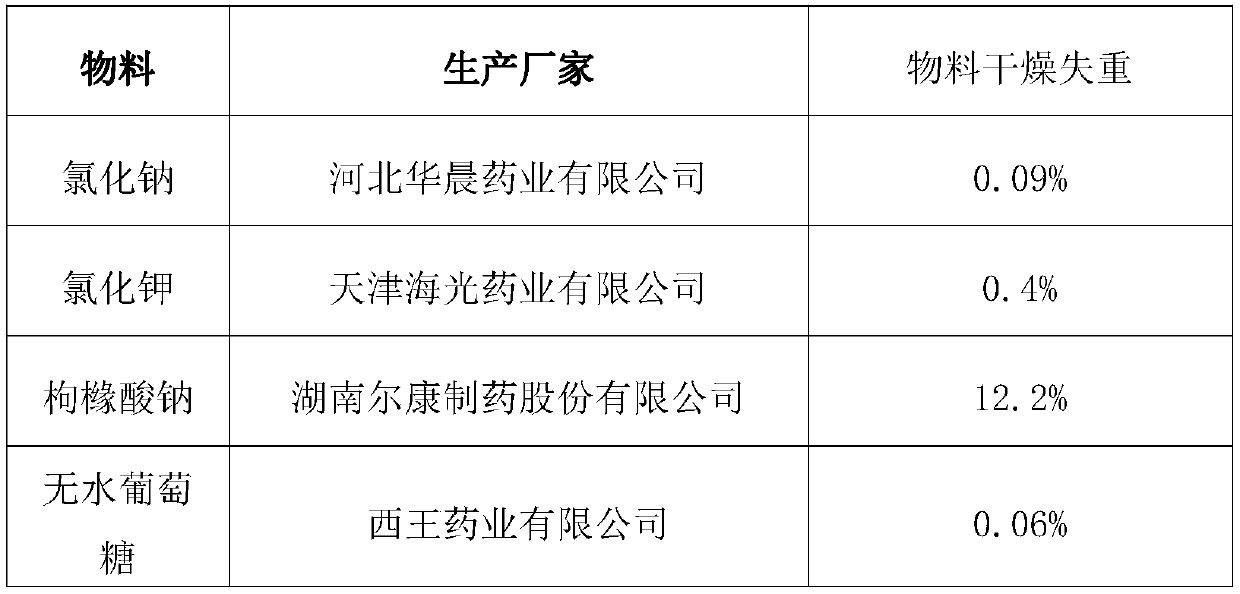
[0027] Embodiment 1: make supplementary salt powder of 5 grams / bag specification, and its consumption of every bag is as follows:
[0028] materials unit dose Sodium chloride 0.65g potassium chloride 0.375g sodium citrate 0.825g anhydrous glucose 3.375g
[0029] After the above materials are dried, they will lose water and weight, so that the whole bag packaging is close to 5g.
Embodiment 2
[0030] Embodiment two: the production specification is 5 grams / bag of supplementing salt powder, and the output is that the consumption of 1000 bags is as follows:
[0031] materials Dosage Sodium chloride 0.6506kg potassium chloride 0.3765kg sodium citrate 0.8257kg anhydrous glucose 3.3777kg
[0032] After the above materials are dried, they will lose water and lose weight, and they will be sub-packaged after processing, so that the packaging in small bags will approach 5g after sub-packaging.
Embodiment 3
[0033] Embodiment 3: The preparation method of oral rehydration salt powder.
[0034] 1. Crushing process
[0035] Take sodium chloride, potassium chloride, and sodium citrate respectively, and put them on a 30B universal grinder with an 80-mesh sieve to grind them separately, set aside, calculate the loss rate, and use a fast moisture meter to measure the moisture content of the crushed material to determine whether subsequent drying is required .
[0036] 2. Mixing process
[0037] (1) Equipment / Consumables
[0038] name factory model 3D Motion Mixer Changzhou Xuanyu Drying Equipment Co., Ltd. SYH-10
[0039] (2) mixed
[0040] Put the weighed 35% anhydrous glucose into the mixer, add the weighed sodium chloride, potassium chloride and sodium citrate in the prescribed amount, put each material into the three-dimensional motion mixer in sequence, close the feeding port, and start The mixer was turned on and mixed for 10 minutes. Observing the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com