Nickel catalyst with high steric hindrance, synthesis method therefor and application of nickel catalyst
A technology of nickel catalyst and synthesis method, applied in nickel organic compounds, chemical instruments and methods, compounds containing elements of Group 8/9/10/18 of the periodic table, etc. Limitation, insufficient catalyst activity, etc., to achieve the effect of increasing instability, high regularity, and reducing the rate of transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
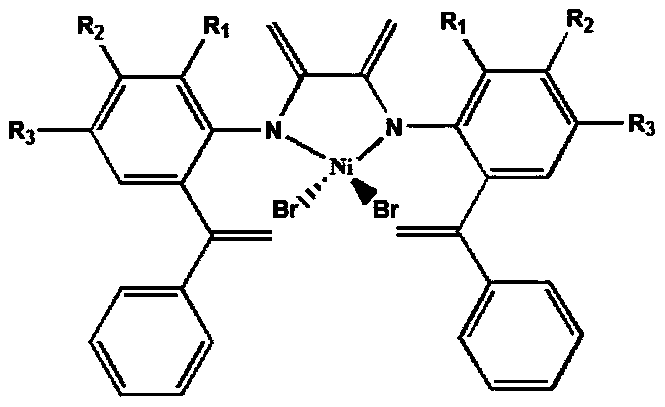
[0023] A structure is a large steric hindered nickel catalyst of α-diimine nickel (II) substituted by an ortho-phenyl vinyl group, and its synthesis method is as follows:
[0024] (1) Synthesis of 2,3-dimethyl-6-phenylvinylaniline: 2,3-dimethylaniline (0.24g, 2.00mmol), styrene (0.52g, 5.00mmol), xylene (2mL), trifluoromethanesulfonic acid (0.06g, 0.4mmol) were sequentially added to a 100mL Schlenk bottle, reacted at 260°C for 48h, and the residue obtained was washed three times with ether (3×10mL) to remove volatile substances , and then separated by column chromatography with petroleum ether / ethyl acetate (volume / volume=30:1), to obtain 0.28 g (62% yield) of a light yellow liquid whose structure is 2,3-dimethyl-6 -Phenylvinylaniline. The specific reaction principle is as follows.
[0025]
[0026] (2) Synthesis of ligands: 2,3-dimethyl-6-phenylvinylaniline (0.49g, 2.2mmol) and diacetyl (0.087g, 1.00mmol) were added to a 100mL round bottom flask, Then add 40mL of absolu...
Embodiment 2
[0033] Materials and reagents in Example 1: All organometallic reactions were carried out under N 2 Carry out under protection; Solvent all needs to dry deoxygenation treatment; O-dichlorobenzene (analytical pure) and dichloromethane, use Molecular sieve pre-dried and then in N 2 CaH 2 Reflux, evaporate before use; high-purity N 2 and polymer grade ethylene monomer, deoxygenated and dried before use; toluene, ether (analytical pure), after molecular sieve dehydration, added metal sodium to reflux under nitrogen protection, and steamed before use; anhydrous methanol, ethanol, chloroform ( Analytical pure), used directly; DME (1,2-dimethoxyethane) (analytical pure), used Molecular sieve drying; 2,3-dimethylaniline, styrene and trifluoromethanesulfonic acid are all products of Aladdin Company, and diethylaluminum chloride (0.9M toluene solution) is a product of Aldrich Company.
Embodiment 3
[0035] A large sterically hindered nickel catalyst with a structure of α-diimine nickel (II) substituted by an ortho-phenylvinyl group is used as a late transition metal catalyst for the polymerization of ethylene. The specific polymerization process is as follows.
[0036] The 250mL Schlenk polymerization bottle with a magnetic stirring bar was subjected to vacuum-nitrogen cycle replacement three times. Under a nitrogen atmosphere, 50mL of toluene dehydrated and deoxygenated by metal sodium reflux was injected with a syringe, and then ethylene was introduced to fully absorb ethylene until it was saturated. Add 3.5mL (0.9M) cocatalyst DEAC (diethylaluminum chloride) into the reaction flask with a syringe, stir at 60°C for 15 minutes, and then use a syringe to dissolve 5mmol of 1 nickel catalyst, maintain the pressure required for ethylene polymerization (0.2bar), and continue to polymerize at 60°C for 10 minutes; then use 100mL of 5% to 95% hydrochloric acid-methanol solution t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com