Chemical semi-synthesis method of artemisinin
An artemisinin, semi-synthetic technology, applied in the directions of organic chemistry, chemical instruments and methods, organic chemistry, etc., can solve the problems of low total yield of artemisinin, unsuitable for industrial production, uneven reaction illumination, etc. The effect of photooxidation reaction, inhibition of decarboxylation side reaction, process safety and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 2
[0074] Preparation example The preparation of dihydroartemisinic acid
[0075] Add 3000ml of absolute ethanol and 2000ml (35.02mol) of 85% hydrazine hydrate to 1000g (4.2675mol) of artemisinic acid, add 1800ml (17.6294mol) of 30% hydrogen peroxide dropwise at -10°C under temperature control, after 4H the reaction is complete, and add 6N dropwise hydrochloric acid aqueous solution to pH=1 to obtain 996.6 g (4.2170 mol) of dihydroartemisinic acid, yield 98.74%.
Embodiment 1
[0076] Embodiment 1: the preparation of artemisinin
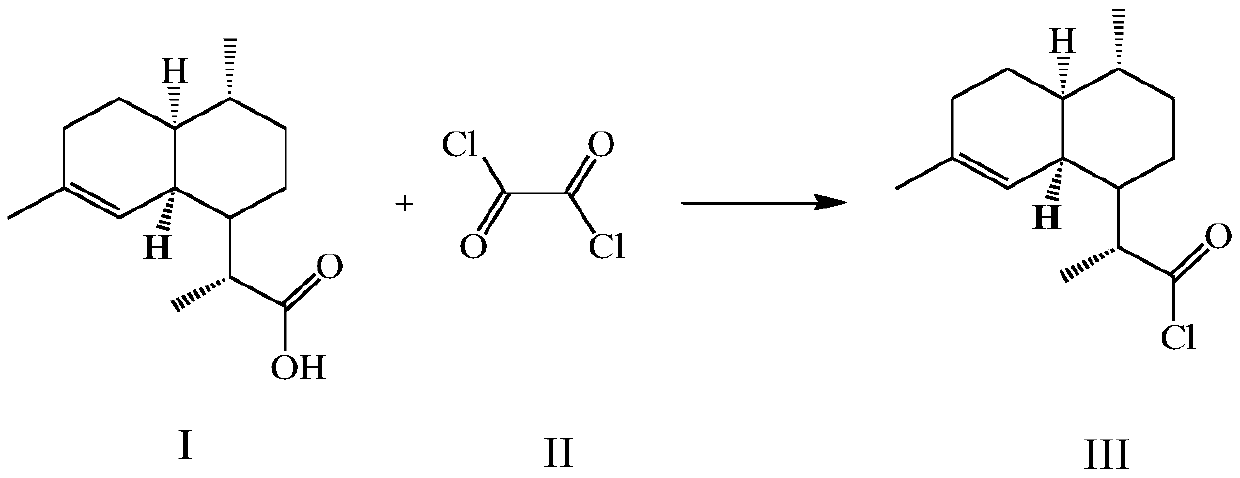
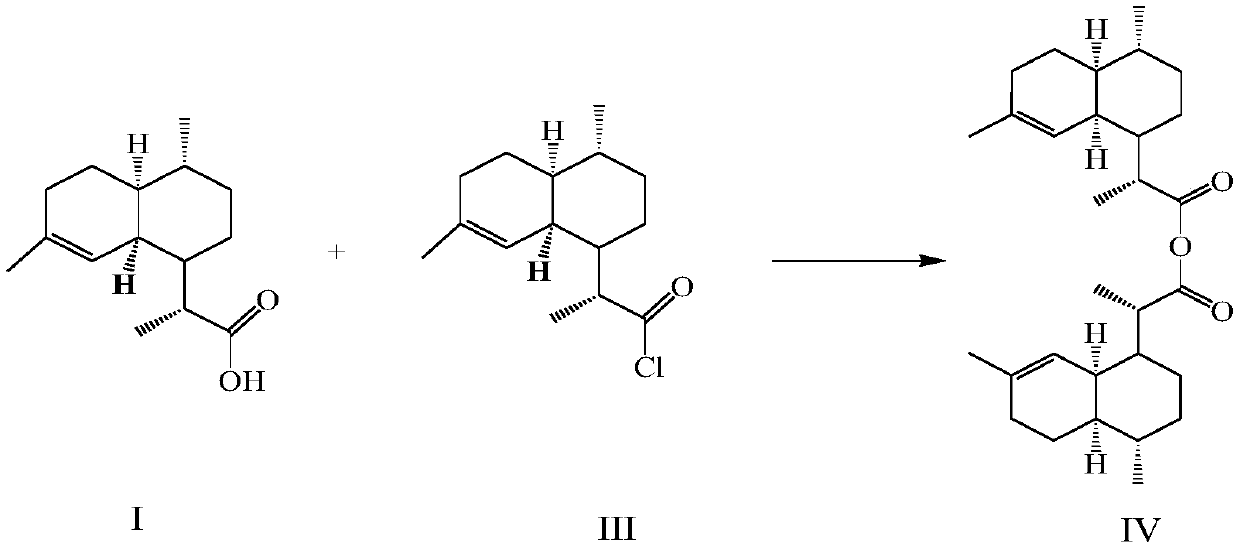
[0077] 50g (0.2116mol) of dihydroartemisinic acid prepared in the preparation example was added to 250ml of dichloromethane, 2.5ml (0.03247mol) of N,N-dimethylformamide was added, and 21.67ml ( 0.2543mol) oxalyl chloride, the reaction was completed after 1 hour, and the reaction solution was concentrated to dryness to obtain 53.2g (0.2094mol) of dihydroartemisinic acid chloride, then added 250ml dichloromethane, 20ml triethylamine, and added dihydroartemisinic acid chloride at 0°C 50 g (0.2116 mol) of hydroartemisinic acid, after 2 hours, the reaction was completed, and concentrated to obtain 94.11 g (0.2073 mol) of dihydroartemisinic anhydride, with a yield of 97.97%.
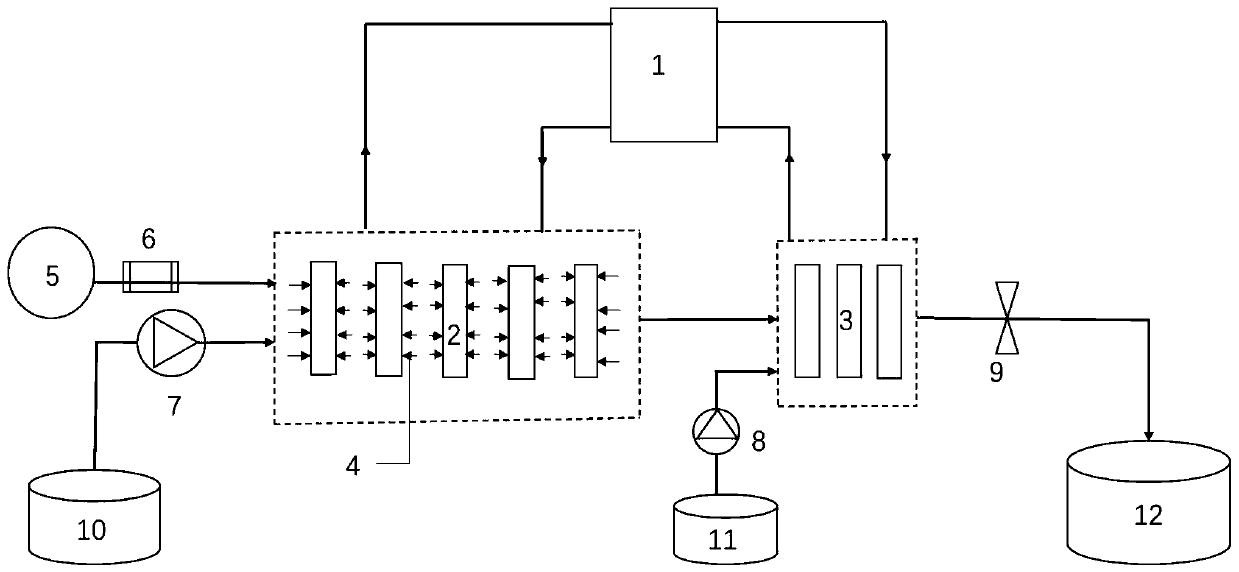
[0078] 94.11 g (0.2073 mol) of dihydroartemisinic anhydride and 0.3 g tetraphenylporphyrin (0.00049 mol) were added to dissolve in 560 ml of dichloromethane to obtain a feed solution. Set the temperature connected to the glass microchannel module 2 in the ...
Embodiment 2
[0080] Embodiment 2: the preparation of artemisinin
[0081] 50g (0.2116mol) of dihydroartemisinic acid prepared in the preparation example was added to 250ml of dichloromethane, 2.5ml (0.03247mol) of N,N-dimethylformamide was added, and 21.67ml ( 0.2543mol) of oxalyl chloride, the reaction was completed after 1 hour, the reaction solution was concentrated to dryness to obtain 52.8g (0.2079mol) of dihydroartemisinic acid chloride, then 250ml of dichloromethane and 20ml of triethylamine were added, and the temperature was controlled at -5°C. 50 g (0.2116 mol) of dihydroartemisinic acid, after 2 hours the reaction was completed, and concentrated to obtain 93.1 g (0.2051 mol) of dihydroartemisinic anhydride, with a yield of 96.93%.
[0082] 93.1 g (0.2051 mol) of dihydroartemisinic anhydride and 1.3 g tetraphenylporphyrin (0.002117 mol) were dissolved in 370 ml of toluene to obtain a liquid. Set the temperature connected to the glass microchannel module 2 in the temperature cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com