Preparation method and application of HAO rabbit polyclonal antibody
A technology of polyclonal antibody and antibody serum, applied in the direction of anti-enzyme immunoglobulin, immunoglobulin from serum, measuring devices, etc., can solve the problems of inability to detect and express HAO expression, undisclosed HAO antibody preparation methods, etc., to achieve The effect of improving detection efficiency and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of HAO recombinant protein
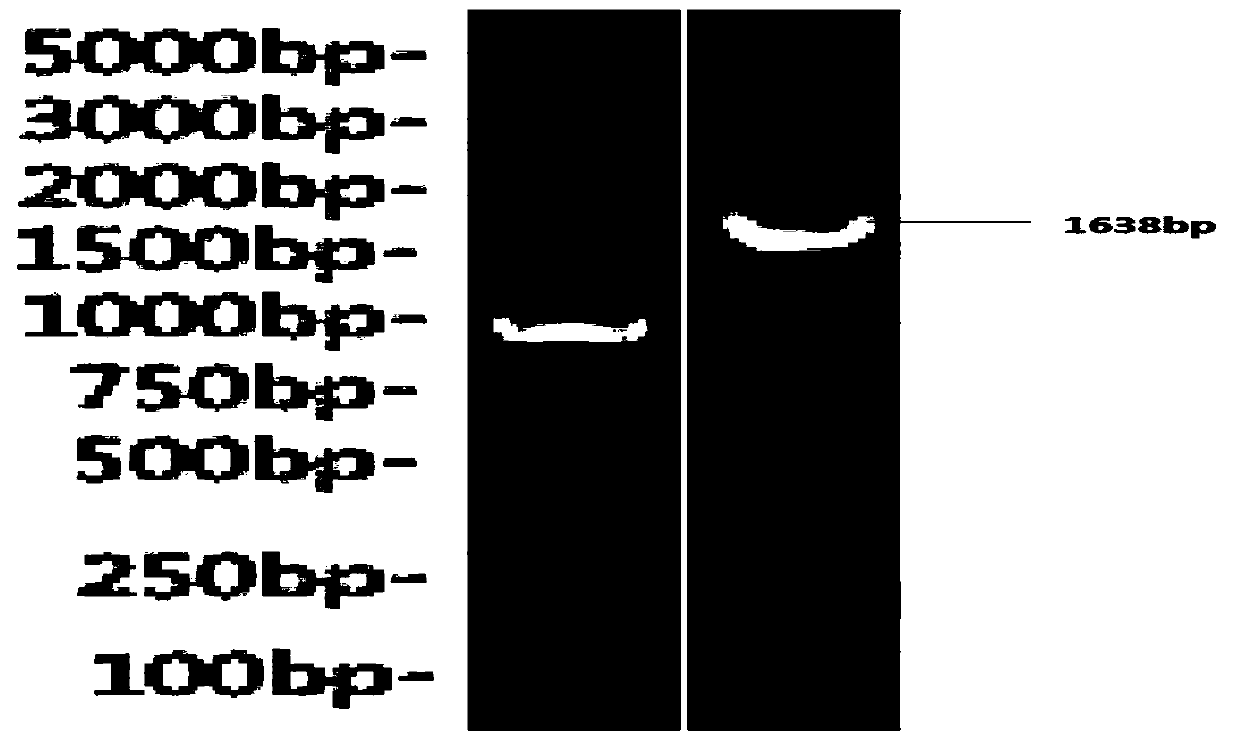
[0032] Using the ATCC 19718 culture as a template, the nucleic acid sequence of Hydroxylamine oxidoreductase 25-570 amino acids was amplified by PCR with Pfu high-fidelity DNA polymerase (Tiangen ep101), the fragment length was 1638bp, identified by 1% agarose gel electrophoresis, see figure 1 ; Use the DNA product recovery kit (Axygen, AP-GX-250) to recover the target band by cutting the gel, and the recovered product is digested with restriction endonucleases Bam HI and XhoI, and the gel is recovered and the same enzyme digestion and gel recovery are carried out. The treated pET28a(+)-SUMO plasmid fragments were ligated overnight at 4°C, transformed into DH5α competent cells (Tiangen), picked clones, and after PCR identification and sequencing confirmed positive, plasmid DNA mini-extraction kit (Axygen, AP-MN-P-250) to extract the plasmid and carry out the next step of transformation. The resulting plasmid was transf...
Embodiment 2
[0033] Example 2 Identification of protein expression for immunization
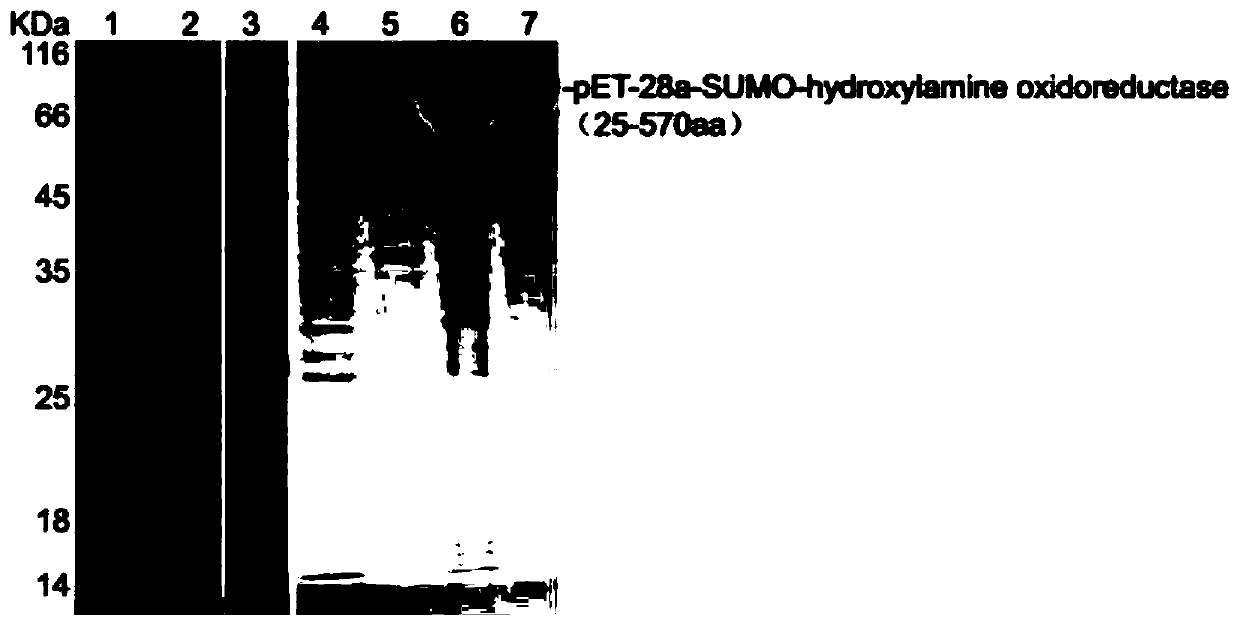
[0034] 4000rpm centrifugation 10min of the expression bacterial strain that has expressed the protein of interest obtained in embodiment 1 is collected thalline and discards supernatant, phosphate buffered saline (Solei Bao) resuspended thallus, add the β-mercaptoethanol of 1 / 1000 volume ( Sigma-Aldrich), put the bacterial solution in ice cubes, crush the bacterial cells with an ultrasonic breaker, the power is 400W, the ultrasonic time is 3s and the interval is 3s for a total of 10min, and the bacterial supernatant 1 and inclusion body precipitation are obtained by centrifugation at 9000rpm for 10min. 2M urea solution was added to the precipitate, ultrasonicated under the same conditions and then centrifuged to obtain supernatant 2 and inclusion body precipitate. 8M urea solution was used to dissolve the inclusion body precipitate, and centrifuged at 12000rpm for 1min to obtain the supernatant as the inc...
Embodiment 3
[0035] Example 3 Antiserum Affinity Purification Using Protein Detection
[0036]The process of Example 1 and Example 2 was repeated to obtain an inclusion body protein solution, which was stained with Coomassie Brilliant Blue after SDS-PAGE electrophoresis. The results showed that the protein concentration was 2 mg / ml, and the purity was equivalent to that of the antigen protein, which could be used for antigen affinity purification. see results image 3 ;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com