Algin oligosaccharide diacid composition
A technology of algin oligosaccharide and composition, which is applied in the field of algin oligosaccharide diacid composition, and can solve the problem of high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] Preparation method of algin oligosaccharide diacid composition
[0063] The preparation process of algin oligosaccharide diacid of the present invention is summarized as follows:
[0064] After preliminary degradation of alginic acid, a mixed polysaccharide of polymannuronic acid and polyguluronic acid can be obtained, and the mixed polysaccharide can be precipitated by acid method to remove a certain amount of polyguluronic acid; The higher the pH value control, the higher the polyguluronic acid content in the obtained mixed polysaccharide. For example, refer to the methods disclosed in Chinese Patent Application No.98806637.8 and CN02823707.2. In the presence of an oxidizing agent, the mixed polysaccharides described above undergo oxidative degradation of sugar chains to obtain oxidized oligosaccharides with different degrees of polymerization. The oxidized oligosaccharides are characterized in that mannuronic acid or guluronic acid at the reducing end of the oligo...
Embodiment 1
[0164] Step 1): Preparation of algin oligosaccharide mixture
[0165] Make a 10% solution of 5Kg sodium alginate, add dilute hydrochloric acid to adjust the pH to about 3.0, raise the temperature to 80°C, stir, react for 10 hours, stop heating, cool to room temperature, add NaOH to adjust the pH to 9.0, then add Adjust the pH to 3.2 with dilute hydrochloric acid, centrifuge at 5000 rpm for 10 min, collect the supernatant, concentrate with a rotary evaporator, and dry in vacuum to obtain 1500 g of the intermediate.
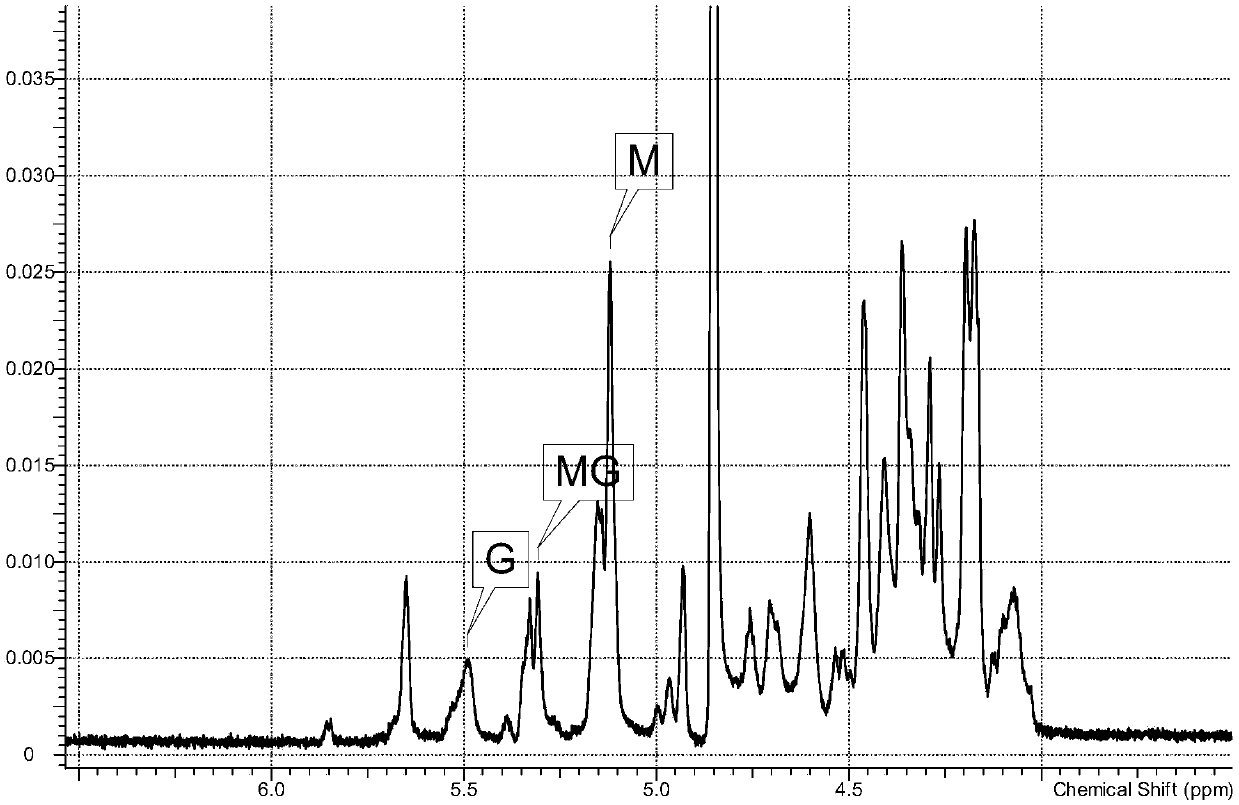
[0166] The NMR spectrum of the intermediate is attached figure 1 . The NMR determination method is as follows: Sample preparation: Weigh 30 mg of the sample to be tested and dissolve it in 0.5 ml D2O, freeze-dry, add 0.5 ml deuterated heavy water to dissolve, freeze-dry again, and finally dissolve the freeze-dried sample powder with an appropriate amount of heavy water Transfer to an NMR tube, prepare a 100 mg / ml solution to be tested, and add 0.01% (w / v) deutera...
Embodiment 2
[0191] Weigh 100g of commercially available sodium alginate (purchased by Sinopharm Reagent Network, CAS No. 9005-38-3, specification CP, Shanghai test), add distilled water and mix evenly, after swelling, make a solution with a volume of 1L, and adjust the pH to 4.0 with NaOH , Reaction at room temperature 25°C. Adjust the gas flow at the outlet of the oxygen cylinder and the power of the ozone generator so that the flow rate of the ozone mass concentration reaches 1g / hr, and it is passed into the reaction solution. Stop feeding ozone after reacting for 10 hours, add appropriate amount of water to adjust the concentration of the solution to about 15%, filter with an ultrafiltration membrane with a molecular weight cut-off of 1000 Da, collect the permeate, concentrate with a rotary evaporator, and dry in vacuo to obtain 80 g of product B.
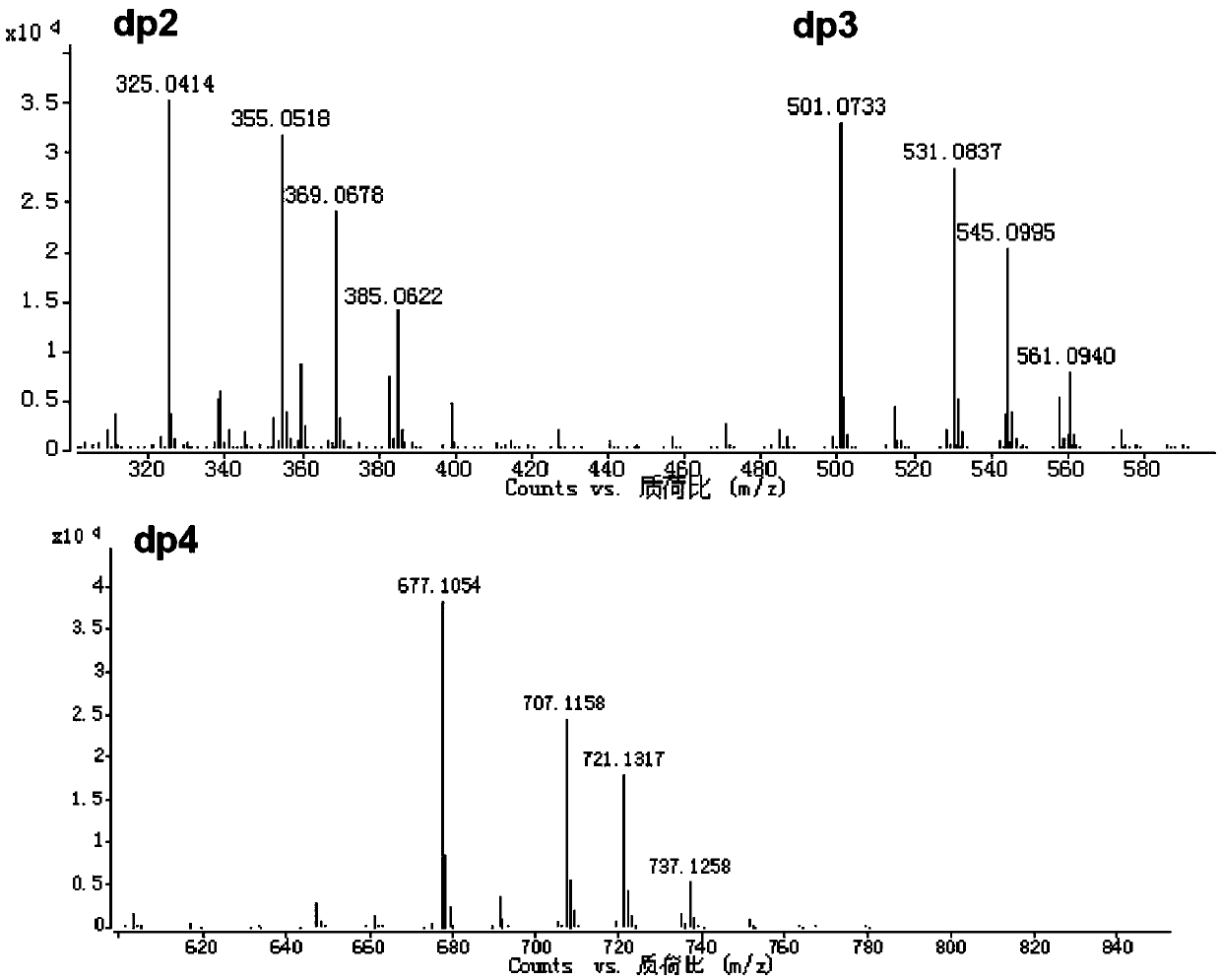
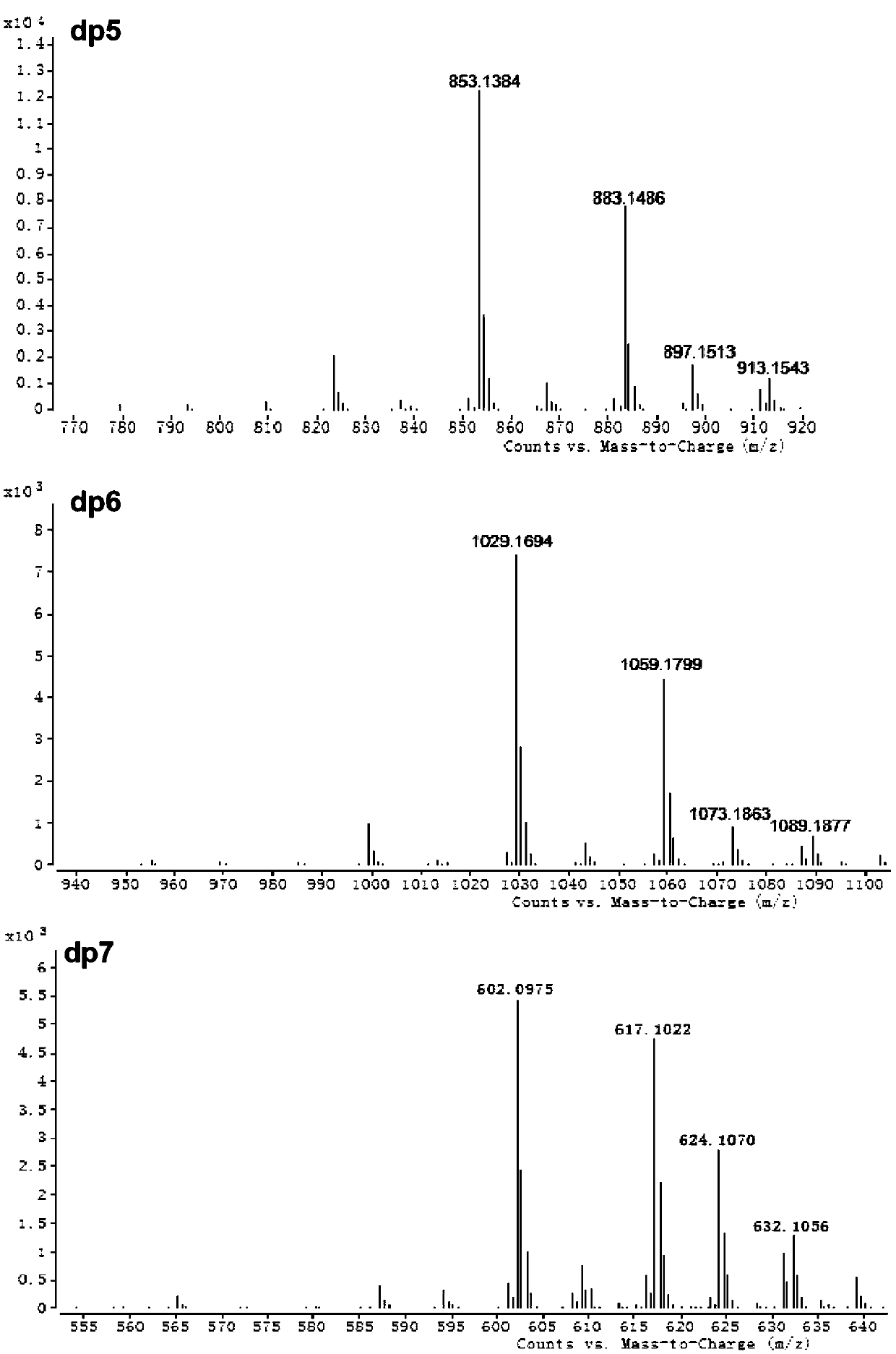
[0192] Superdex peptide (GE Company) size exclusion chromatography combined with multi-angle laser light scattering (MALS, Wyatt Company) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com