Synthesis method of rivastigmine optical isomerization intermediate and (R)-rivastigmine
A technology of optical isomerization and synthesis method, which is applied in the field of chiral drug synthesis in pharmaceutical and chemical industry, which can solve the problems of long synthesis route, low total yield, unfriendliness, etc., and achieve the effect of short synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
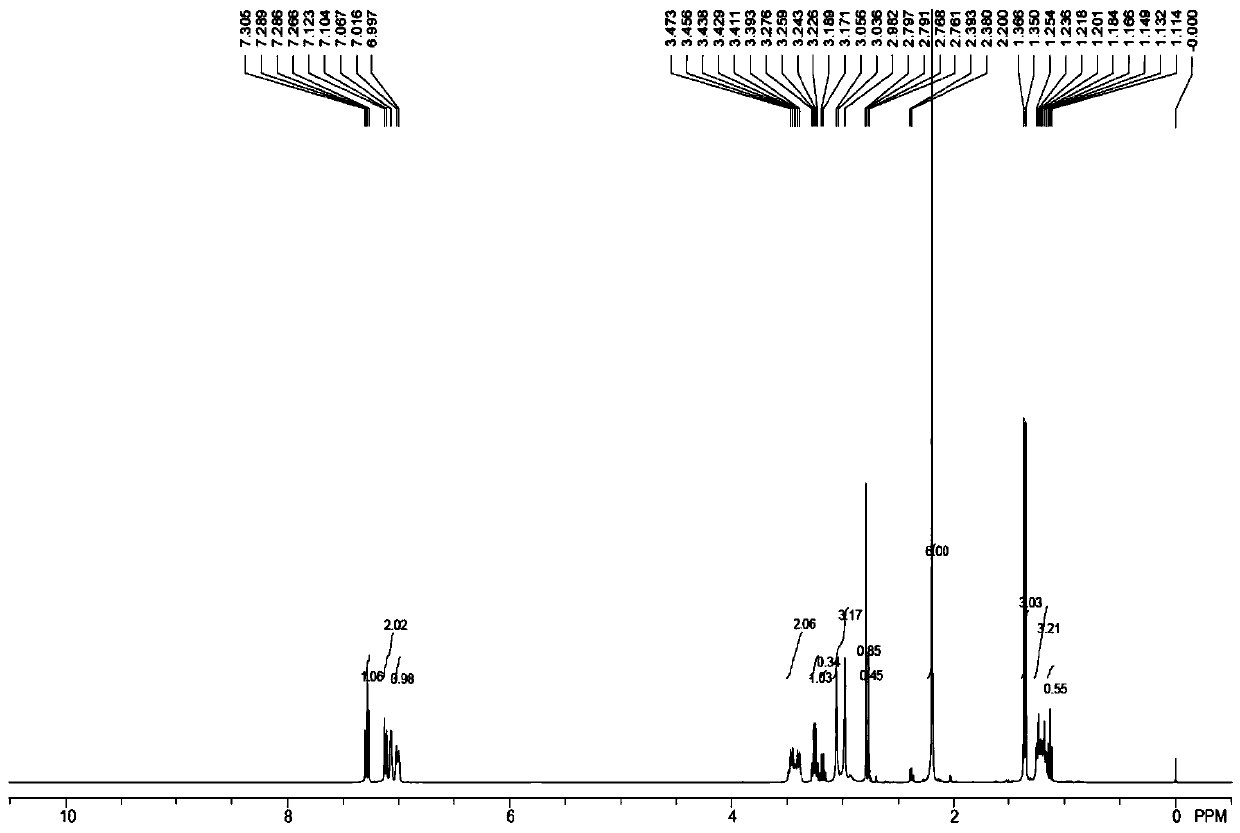
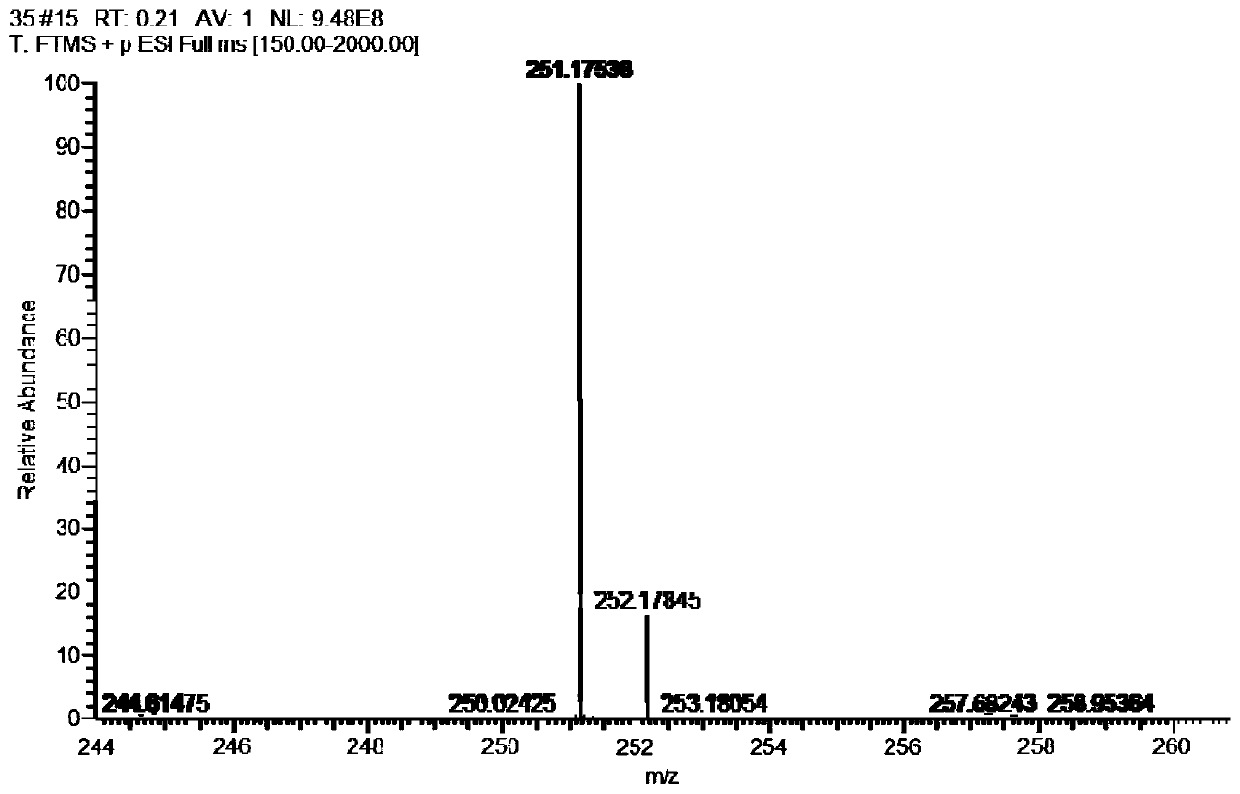
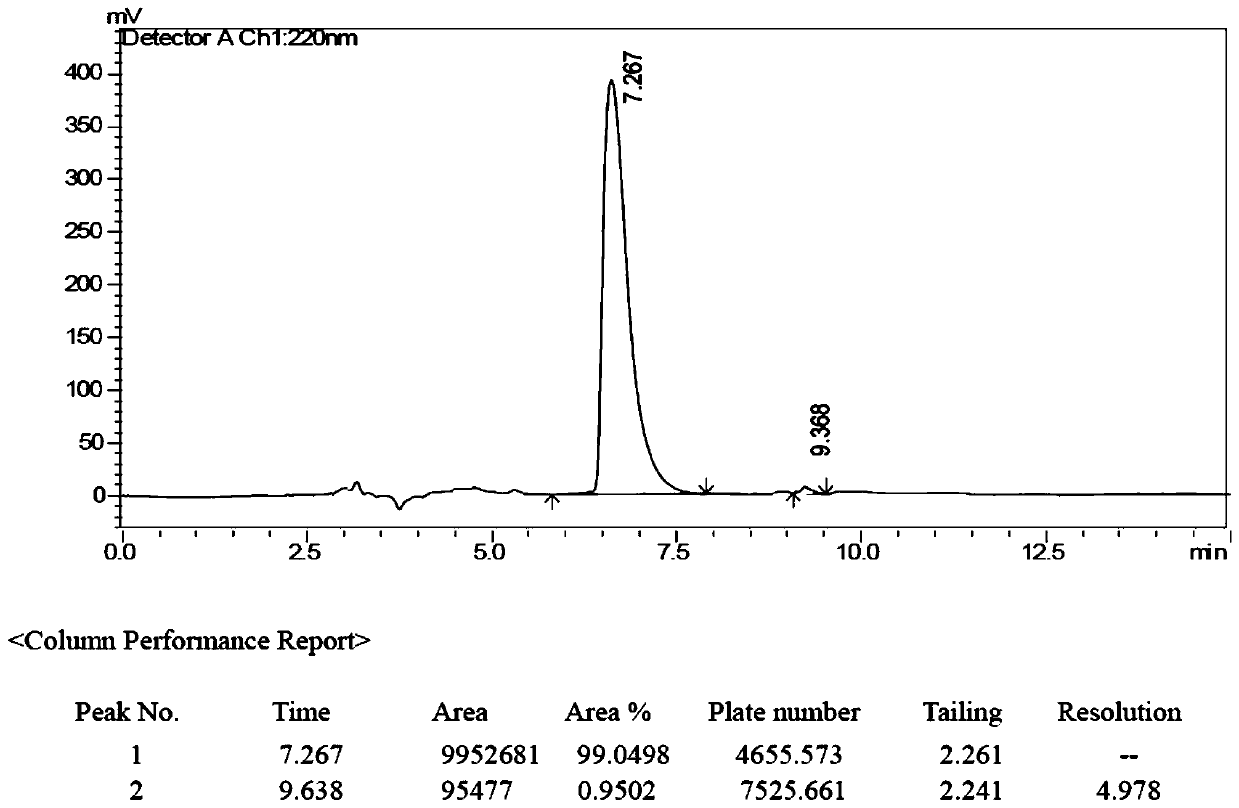
Image
Examples
Embodiment approach
[0029] A typical embodiment of the present disclosure provides a method for synthesizing the optical isomerization intermediate of rivastigmine, which is catalyzed by 3-nitromethyl ethyl formate acetophenone and benzhydrylamine under the catalysis of Ir complex Obtained by asymmetric reductive amination reaction;
[0030] The chemical structural formula of 3-nitromethyl ethyl formate acetophenone (compound 1) is:
[0031] The chemical structural formula of rivastigmine optical isomerism intermediate (compound 2) is:
[0032] The chemical structural formula of the Ir complex is:
[0033]
[0034] The present disclosure finds through experiments that the optical isomerization intermediate of rivastigmine can be prepared by using the above-mentioned Ir complex, which has the advantages of high selectivity and high yield.
[0035] In one or more examples of this embodiment, the step of asymmetric reductive amination reaction is: adding organic acid, titanic acid to 3-nitr...
Embodiment 1
[0075] (1) Synthesis of N-methylethyl (3-acetylphenyl) carbamate (compound 1):
[0076] Add pretreated 100mL of anhydrous dichloromethane into a 250mL three-necked flask, add 10g of 3-hydroxyacetophenone and 15g of methyl ethyl carbamoyl chloride in turn under stirring, then add 10g of finely ground anhydrous sodium carbonate in batches, and finish adding Afterwards, the temperature was raised to 40°C until TLC showed that the 3-hydroxyacetophenone point basically disappeared (developing agent: PE / EA=4 / 1, and the reaction took about 8 hours). After removing the solvent, N-methylethyl(3-acetylphenyl)carbamate was obtained as light yellow oil (based on 3-hydroxyacetophenone, the yield was 99%).
[0077] (2) Synthesis of (R)-3-(1-(phenylamino)ethyl)phenylethyl (methyl)carbamate (compound 2):
[0078] Add 10mL of pre-anhydrous dichloromethane to the 25mL single-necked bottle, add 221mg N-methylethyl (3-acetylphenyl) carbamate and 240mg benzhydrylamine successively under stirring,...
Embodiment 2
[0084] (1) Synthesis of N-methylethyl (3-acetylphenyl) carbamate (compound 1):
[0085] Add 100mL of pretreated anhydrous toluene into a 250mL three-necked flask, add 10g of 3-hydroxyacetophenone and 15g of methyl ethyl carbamoyl chloride in turn under stirring, and then add 10g of finely ground anhydrous sodium bicarbonate in batches, after the addition Raise the temperature to 80°C and react until TLC shows that the 3-hydroxyacetophenone point basically disappears (developer: PE / EA=4 / 1, the reaction takes about 6 hours), the reaction solution is cooled to room temperature and filtered, and the filtrate is evaporated under reduced pressure After solvent, N-methylethyl (3-acetylphenyl) carbamate was obtained as light yellow oil (based on 3-hydroxyacetophenone, the yield was 98%).
[0086] (2) Synthesis of (R)-3-(1-(phenylamino)ethyl)phenylethyl(methyl)carbamate (compound 2)
[0087] Add 10mL of pre-anhydrous dichloromethane to the 25mL single-necked bottle, add 221mg N-methyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com