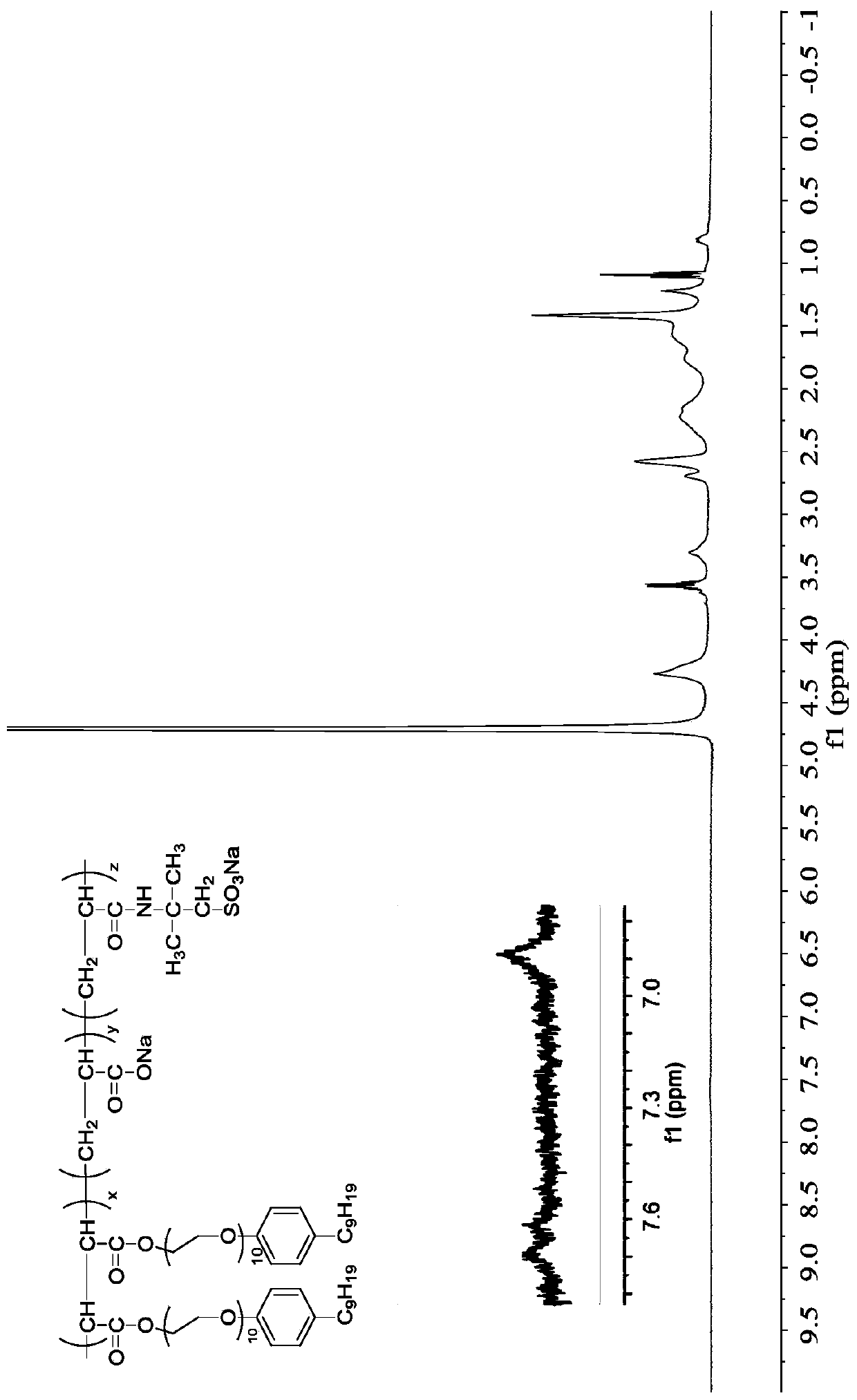
Preparation method and application of double-braid polymer surfactant containing aryl
A technology of surfactant and aromatic polyoxyethylene ether, which is applied in the direction of drilling compositions, chemical instruments and methods, etc., can solve the problems that it is difficult to achieve the purpose of viscosity reduction, difficult to form, and unable to reduce the viscosity of heavy oil. , to achieve the effect of improving oil-water mobility ratio, convenient storage and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] (1) Add nonylphenol polyoxyethylene ether (n=10) (10.0000g, 15.131mmol ), maleic anhydride (0.3810g, 3.886mmol), p-toluenesulfonic acid (0.1556g, 0.902mmol) and toluene (50mL), stirring and dissolving, heating to reflux, maintaining the temperature of the reaction solution at 115-120°C, and continuing the reaction for 6h , to divide the generated water.
[0062] (2) After the reaction is over, the reaction solution obtained in step (1) is washed with 5wt% sodium bicarbonate aqueous solution and saturated sodium chloride aqueous solution respectively, and after concentration, 4.36 g of light yellow oily matter is obtained, which is a compound containing 10 ethyleneoxy groups. Dinonylphenol polyoxyethylene ether maleate diester (NP) of EO unit 10 MA).
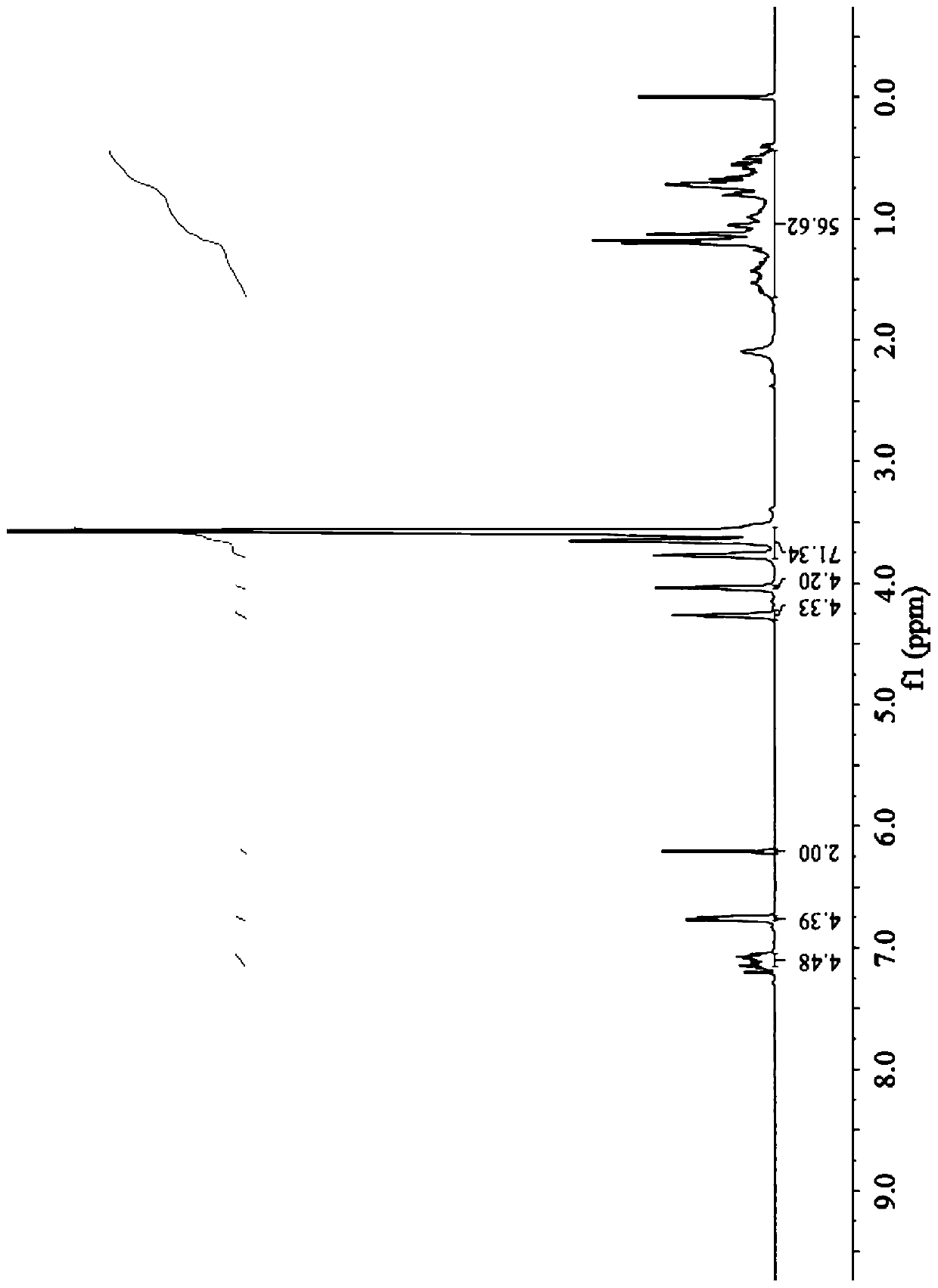
[0063] The proton nuclear magnetic resonance spectrum of the yellow oil obtained in the present embodiment ( 1 HNMR, 400MHz, CDCl 3 )Such as figure 1 As shown, the chemical shift δ (ppm) is 6.70-7.3 (8H, hydrogen on t...
Embodiment 2
[0066] The preparation method is the same as in Example 1, except that: in step (1), nonylphenol polyoxyethylene ether (n=6) (10.0000g, 20.633mmol) containing 6 ethyleneoxy EO units is added, Maleic anhydride (0.9196 g, 9.378 mmol) and p-toluenesulfonic acid (0.1973 g, 1.146 mmol).
[0067] The obtained yellow oil is dinonylphenol polyoxyethylene ether maleic acid diester (NP) containing 6 ethyleneoxy EO units. 6 MA) 7.90 g.
[0068] Its proton nuclear magnetic resonance spectrum data are as follows:
[0069] 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 6.70-7.3 (8H, hydrogen on the benzene ring), 6.25 (2H, hydrogen on the double bond H C=C H ),3.0-4.5(48H, for 12-OC H 2 C H 2 O-), 0.5-2.2 (38H, is alkyl hydrogen).
[0070] Through proton nuclear magnetic resonance spectrum analysis, confirm that the product that obtains is the dinonylphenol polyoxyethylene ether maleic acid diester containing 6 ethyleneoxy EO units (NP 6 MA).
Embodiment 3
[0072] The preparation method is the same as in Example 1, except that in step (1), di-tert-butylphenoxy polyoxyethylene ether (n=15) (10.0000g , 11.533mmol), maleic anhydride (0.3230g, 3.294mmol) and p-toluenesulfonic acid (0.2064g, 1.200mmol).
[0073] The obtained yellow oil is bis(di-tert-butylphenoxypolyoxyethylene ether) maleic acid diester (DPP) containing 15 ethyleneoxy EO chain members. 15 MA) 4.76g.
[0074] Its proton nuclear magnetic resonance spectrum data are as follows:
[0075] 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 6.70-7.3 (8H, hydrogen on the benzene ring), 6.25 (2H, hydrogen on the double bond H C=C H ),3.0-4.5(120H, for 30-OC H 2 C H 2 O-)0.5-2.2 (36H, tert-butyl hydrogen).
[0076] Through proton nuclear magnetic resonance spectrum analysis, confirm that the product that obtains is two (di-tert-butylphenoxy polyoxyethylene ether) maleic acid diester (DPP) containing 15 ethyleneoxy EO chain members 15 MA).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal degradation temperature | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com