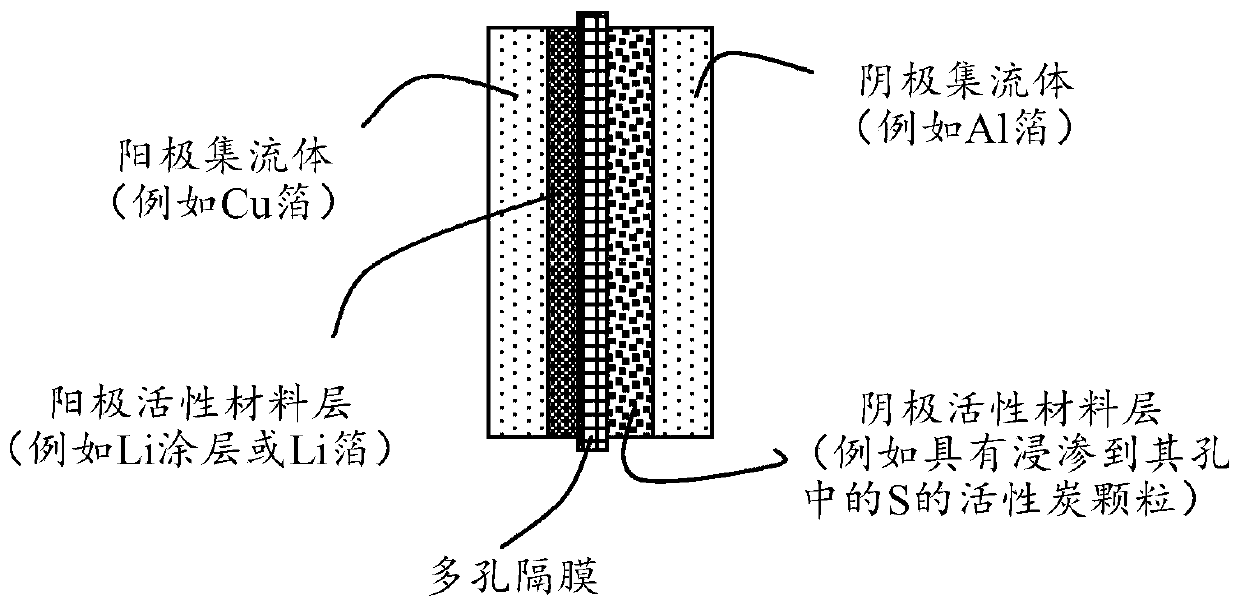
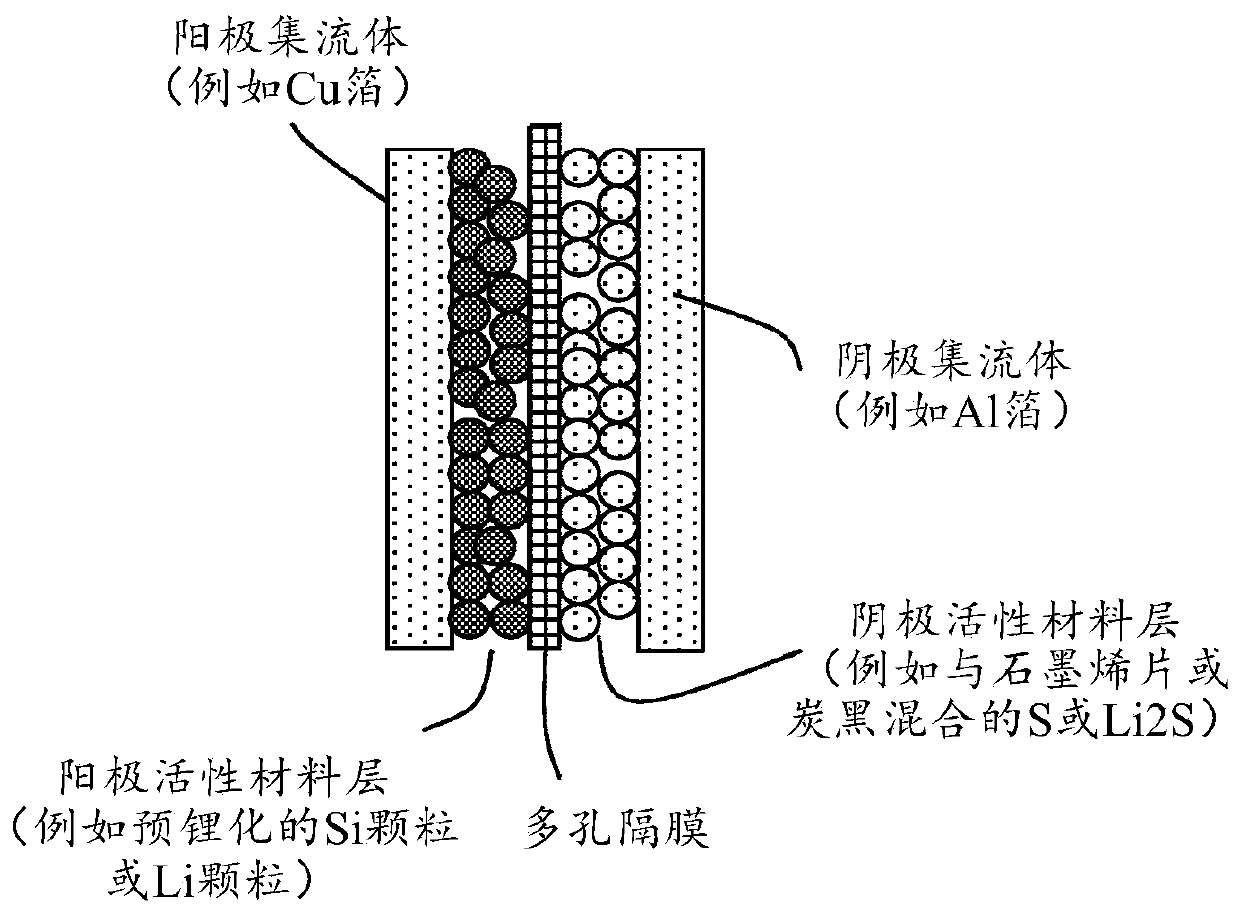
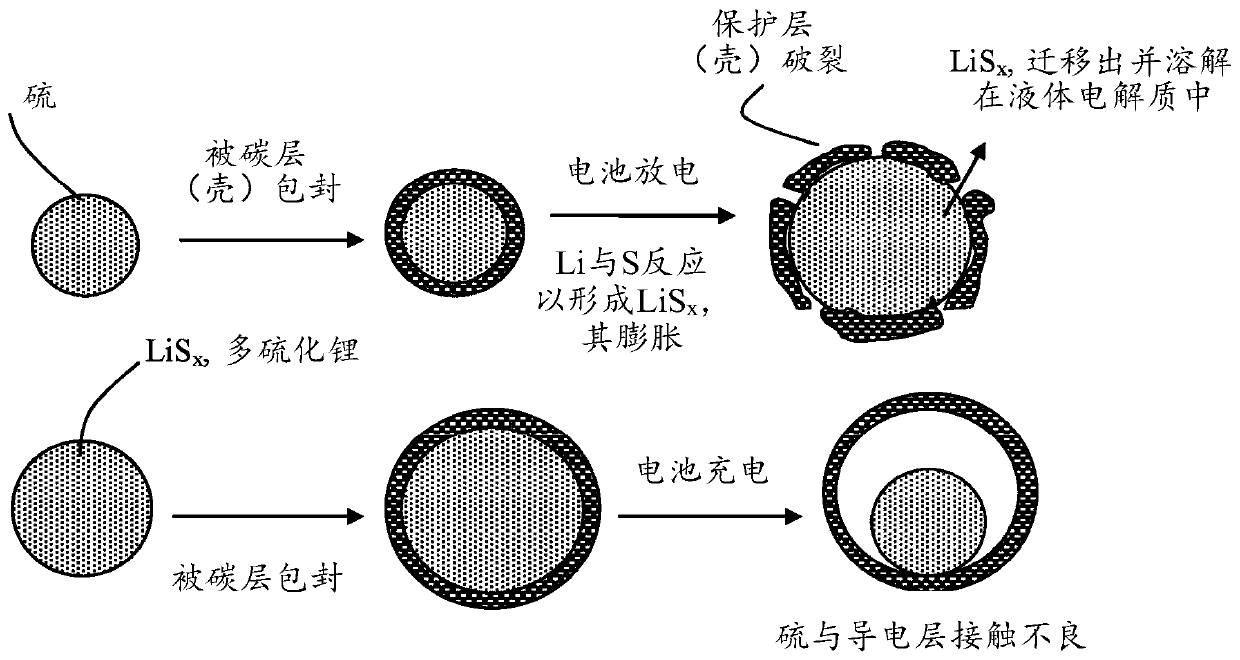
Alkali metal-sulfur secondary battery containing a polymer-encapsulated sulfur cathode and manufacturing method
一种碱金属、聚合物的技术,应用在二次电池、锂蓄电池、电池电极等方向,能够解决短循环寿命、电接触的损失、电芯薄膜隔膜中孔堵塞等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0157] Example 1: Mixing sulfur with carbon / graphite particles by ball milling to form sulfur-containing particles
[0158] Particles of sulfur and lithium polysulfides as well as particles of soft carbon (i.e., graphitizable disordered carbon), natural graphite, mesophase carbon, expanded graphite flakes, carbon nanofibers, and graphene sheets (the resulting composite or hybrid (50% to 85% by weight of S in the compound) were physically blended, and then subjected to ball milling for 2-24 hours to obtain S-containing compound particles (typically in the shape of balls or potatoes). Particles containing various S contents with a typical size of 1-10 μm were then surrounded with a thin layer of highly elastic UHMW polymer (further described later). Some of the resulting particles were then made into a cathode layer.
example 2
[0159] Example 2: Simple sulfur melt or liquid solution mixing
[0160] One way to combine sulfur with conductive materials such as carbon / graphite particles is to use a solution or melt mixing process. Highly porous activated carbon particles, chemically etched mesocarbon microspheres (activated MCMB), and exfoliated graphite worms were mixed with sulfur melt at 117°C–120°C (slightly higher than the melting point of S (115.2°C)) for 10− 60 minutes to obtain sulfur-impregnated carbon particles.
example 3
[0161] Example 3: Preparation of sulfur-coated graphene sheets and their secondary particles (microparticles)
[0162]The steps involve generating a vapor of elemental sulfur that deposits the S vapor on the surface of a single-layer or few-layer graphene sheet. Graphene sheets suspended in a liquid medium (such as graphene oxide in water or graphene in NMP) are sprayed onto a substrate (such as a glass surface) to form a thin layer of graphene sheets. This graphene thin layer is then exposed to sublimation-generated physical vapor deposition. Sublimation of solid sulfur occurs at temperatures greater than 40°C, but meaningful and practically useful sublimation rates typically do not occur until temperatures are greater than 100°C. We typically use 117°C-160°C with a vapor deposition time of 10-120 minutes to deposit a thin film of sulfur (sulfur thickness from about 1 nm to 10 nm) on the graphene surface. This graphene thin layer with a thin film of sulfur deposited thereon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com