Preparation method and application of carbon-based non-noble metal oxygen reduction catalyst
A non-precious metal and catalyst technology, applied in the field of electrocatalysis, carbon-based non-precious metal oxygen reduction catalyst and its preparation, can solve the problems of numerous experimental condition variables, rigid control of effective iron content, complex and time-consuming multi-factor process, etc. Accelerates kinetic processes, facilitates diffusion and fast transport, achieves uniform dispersion and stable loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preparation of polypyrrole doped with sodium ferrocyanide: 0.024mol (11.626g) sodium ferrocyanide decahydrate, 0.016mol (3.648g) ammonium persulfate and 200mL distilled water were added under nitrogen protection In a 500ml three-necked flask, keep the temperature of the system at 0°C and a fixed stirring speed in a refrigerator to dissolve for 10 minutes, dissolve 0.012mol (840μL) of pyrrole in 50mL of absolute ethanol, transfer to the separatory funnel and slowly drop into the three-necked flask , after continuous stirring for 6 hours, the black product was obtained for suction filtration, and washed with distilled water several times during the process, and dried in a vacuum oven at 60°C for 24 hours to obtain sodium ferrocyanide-doped polypyrrole. figure 1 Scanning electron micrographs of polypyrrole.
Embodiment 2
[0022] Example 2: Preparation of carbon-based oxygen reduction catalyst: Take 100 mg of sodium ferrocyanide-doped polypyrrole and 300 mg of sublimed sulfur, use an appropriate amount of absolute ethanol as a wetting agent, grind and mix them in a mortar until uniform. Then put the obtained product in a clean porcelain boat and put it into a high-temperature tube furnace. Under the protection of nitrogen, the temperature was first programmed to rise to 900 °C at 5 °C / min and maintained for 2 hours, and then the temperature was programmatically lowered to 25 °C at 10 °C / min. ℃, the doped carbon material was obtained, named as Fe / 3S / N-C, figure 2 Scanning electron micrographs of the corresponding carbon materials.
Embodiment 3
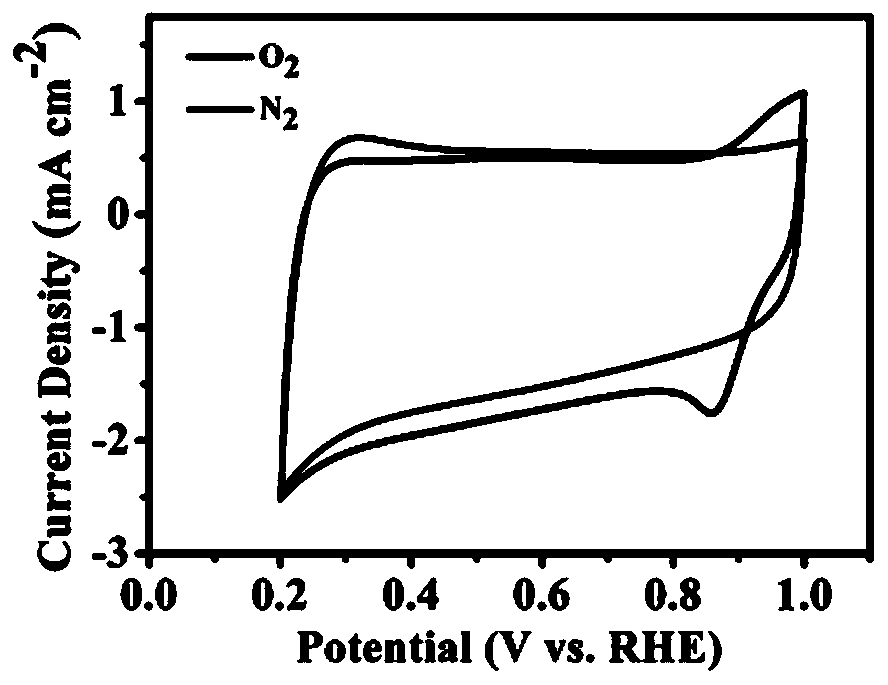
[0023] Example 3: Production of an oxygen reduction working electrode: Disperse 5 mg of the above-mentioned synthesized sample in 800 microliters of Nafion isopropanol solution with a volume fraction of 3%, and disperse the material evenly by ultrasonic waves, and take 10 microliters dropwise in the The dried rotating disc electrode (diameter 5mm), after natural drying, was used to test the electrochemical catalytic performance of the sample. image 3 It is the cyclic voltammetry curve of the oxygen reduction catalyst obtained in this embodiment, in saturated N 2 Under the 0.1M KOH electrolyte solution, the cyclic voltammogram in the voltage range of 0.2-1.0V is similar to a rectangle, and there is no obvious reduction peak. Relatively speaking, at saturation O 2 Under the 0.1M KOH electrolyte solution, there is an obvious characteristic peak of oxygen reduction reaction (ORR), indicating that this material has significant electrocatalytic activity for oxygen reduction reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com