Pyrrole dihydrazone derivative fluorescent probe as well as preparation method and application thereof
A fluorescent probe, pyrrole dihydrazone technology, applied in the field of pyrrole dihydrazone derivatives and its preparation, can solve the problems of complex purification process, limited application, high cost of raw materials, etc., and achieve simple synthesis and post-processing methods and high fluorescence recognition performance , the effect of extensive application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation method of the pyrrole dihydrazone derivative fluorescent probe in this embodiment is as follows:
[0032] 2.79 g (10 mmol) N-morpholine ethyl-2,4-dimethyl-5-formylpyrrole-3-carboxamide and 0.50 g (10 mmol) hydrazine hydrate were dissolved in 0.05 L ethanol, and 0.012 g was added dropwise Acetic acid (0.2 mmol) was used as a catalyst, refluxed and stirred at 80°C for 3-4 hours, cooled to room temperature, filtered under reduced pressure, and the obtained solid was washed with ethanol to obtain the pyrrole dihydrazone derivative fluorescent probe. The yield of the target product was 88%.
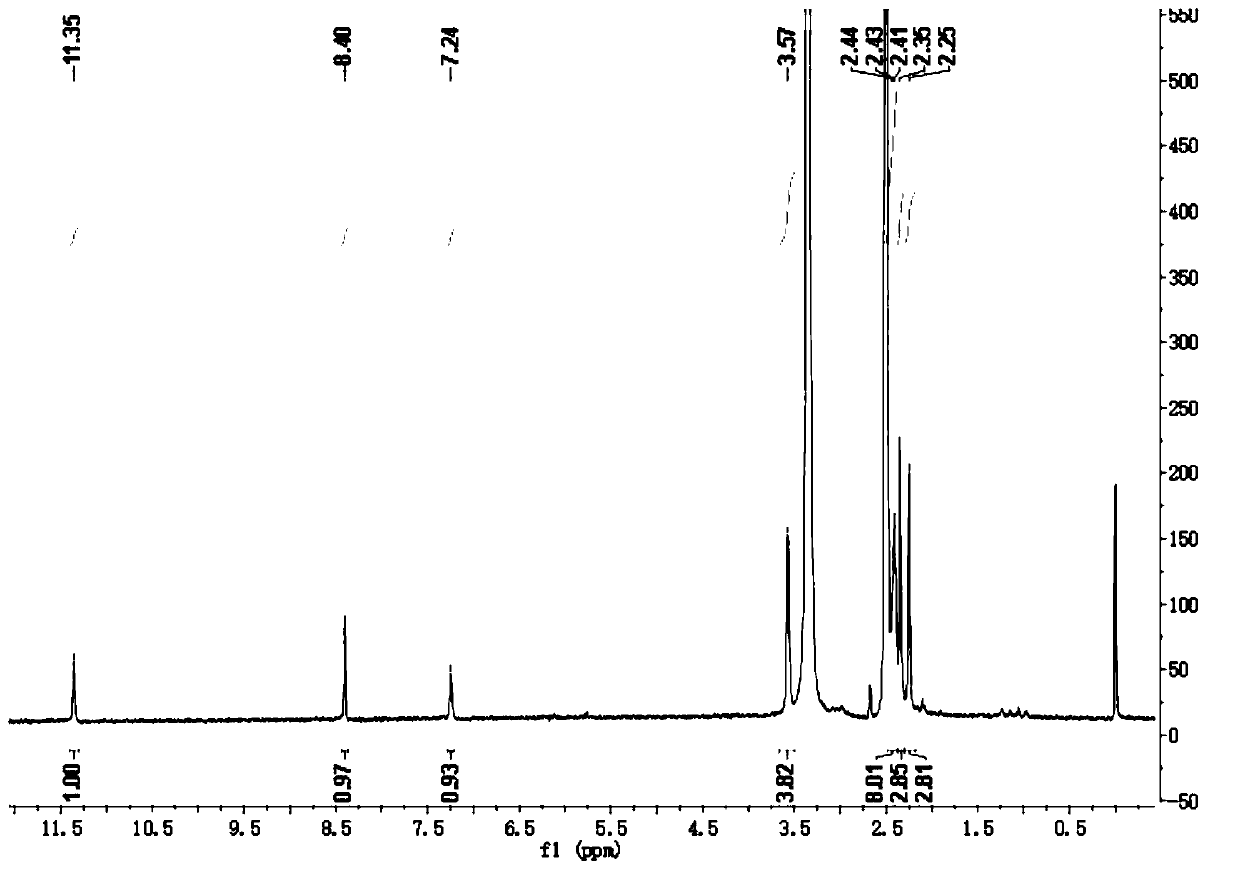
[0033] Adopt nuclear magnetic resonance instrument to carry out nuclear magnetic resonance analysis to the pyrrole dihydrazone derivative that makes, the result is as follows:
[0034] 1 H NMR (400 MHz, DMSO- d 6 ), δ (ppm): 11.35 (s, 1H, NH), 8.40 (s, 1H, CH),7.24 (s, 1H, NH), 3.57 (t, 4H, 2CH 2 ), 2.41-2.44 (m, 8H, 4CH 2 ), 2.35 (s, 3H,CH 3 ), 2.25 (s, 3H, CH 3...
Embodiment 2
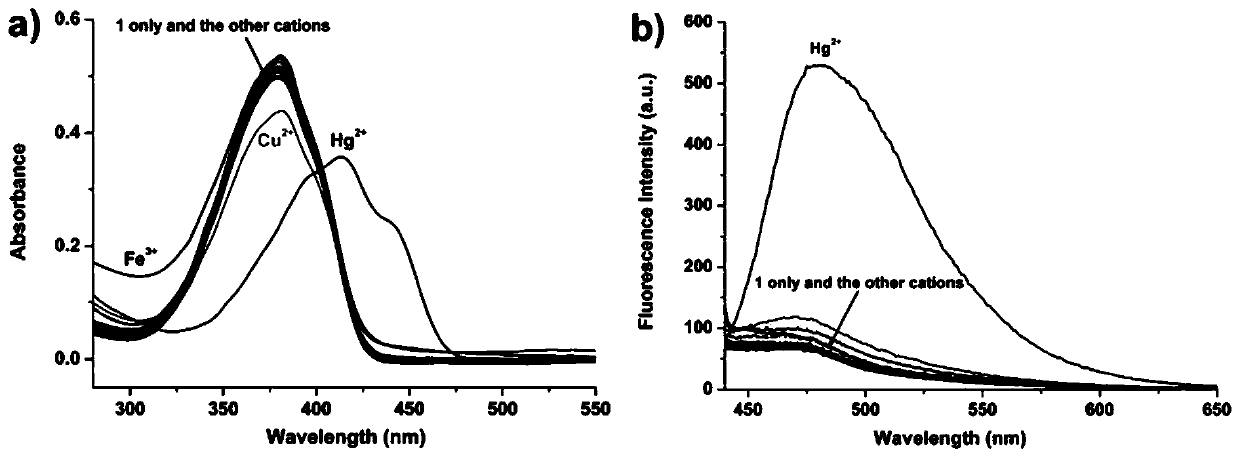
[0037] Determination of Optical Properties of Pyrrole-Containing Dihydrazone Derivatives to Mercury Ions
[0038] The pyrrole dihydrazone derivative prepared in Example 1 above was used as a fluorescent probe in an acetonitrile / HEPES buffer solution (v:v, 7:3, 0.02 mol / L, pH=5) to prepare a molar concentration of 1×10 -5 mol / L solution, respectively, at a molar concentration of 2×10 -5 mol / L metal ion (Ag + , Al 3+ , Ca 2+ , Cd 2+ , Co 2+ , Cr 3+ , Cu 2+ , Fe 3+ , Hg 2+ , K + ,Mg 2+ , Mn 2+ , Na + , Ni 2+ , Pb 2+ and Zn 2+ ) solution, adding an equal amount of the above-mentioned fluorescent probe solution, and using a UV-Vis spectrophotometer or a fluorescence spectrometer for analysis (excitation wavelength is 410 nm), the resulting UV and fluorescence spectra are shown in image 3 . pass image 3 It can be seen that the pyrrole dihydrazone derivatives prepared in the present invention have obvious responses only to mercury ions as probes, and both the ul...
Embodiment 3
[0041] Detection experiment of pyrrole dihydrazone derivative fluorescent probe in intracellular mercury ion
[0042] 1 x 10 for HeLa cells -5 mol / L pyrrole dihydrazone derivative fluorescent probe prepared in Example 1 above was incubated at 37°C for 30 minutes, and Hg 2+ (2×10 -5 mol / L) and then incubated for 30 minutes to obtain the fluorescence imaging image of HeLa cells, as shown in Figure 5 As shown, wherein: a is the fluorescence imaging image of the blue channel of the above-mentioned fluorescent probe; b is the bright-field image of the above-mentioned fluorescent probe; c is the superimposed picture of the above-mentioned fluorescent probe bright-field image and fluorescence image; d is the above-mentioned fluorescence Probe + Hg 2+ Fluorescence imaging image of the blue channel; e is the above fluorescent probe + Hg 2+ Imaging image under bright field; f is the above-mentioned fluorescent probe Hg 2+ Superimposed images of brightfield and fluorescence image...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com