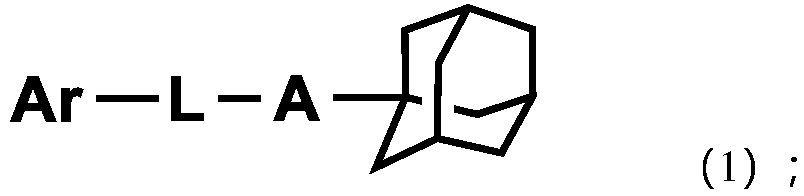
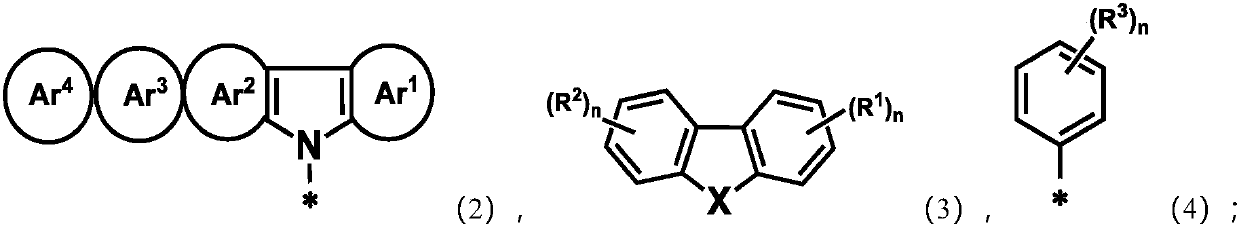
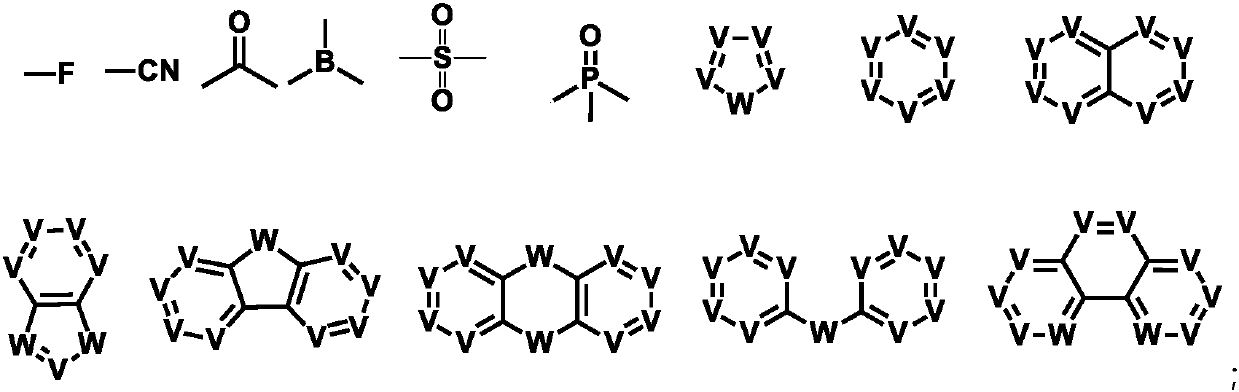
Adamantane-containing compound, high polymer, mixture, composition, and electronic device
A compound, adamantane technology, applied in the field of electroluminescent materials, can solve the problems of small steric hindrance of cyclohexane and limited exciton dispersion, and achieve the effects of reducing interaction, improving energy utilization, and optimizing device performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0236] The synthesis of embodiment 1 compound (M1)
[0237] synthetic route:
[0238]
[0239] 1) Synthesis of intermediate M1-3: under nitrogen atmosphere, (2.88g, 120mmol) magnesium chips, (6-4g, 30mmol) compound M1-2, 3g iodine element and 20mL anhydrous tetrahydrofuran (THF) solvent were added In a 250mL two-necked bottle, heat and stir to initiate the Grignard reaction. After initiation, add (15g, 70mm01) 100mL anhydrous THF solution of compound M1-2 dropwise to the reaction solution, heat at 60°C and continue the reaction for 1 hour. The solution was slowly transferred to a 500mL three-neck flask equipped with (30.1g, 100mmol) M1-1 and 100mL anhydrous THF, reacted at room temperature for 12 hours, cooled to room temperature, quenched with water, and the reaction solution was rotatably evaporated to remove most of the solvent, and used Dichloromethane was dissolved and washed 3 times with water, and the collected organic liquid was mixed with silica gel and passed thr...
Embodiment 2
[0241] The synthesis of embodiment 2 compound (M2)
[0242] synthetic route:
[0243]
[0244] 1) Synthesis of intermediate M2-3: under nitrogen atmosphere, (33.2g, 100mmol) compound M2-1, (28.2g, 100mmol) compound M2-2, (9.55g, 50mmol) cuprous iodide, (5.7 g, 50mmol) trans-cyclohexanediamine, (31.8g, 100mmol) potassium phosphate and 250mL toluene were added to a 500mL three-neck flask, heated and stirred to 110°C for 12 hours, the reaction was completed, cooled to room temperature, and the filtrate was suction filtered , most of the solvent was evaporated by rotary evaporation, and washed three times with dichloromethane dissolved in water, and the organic liquid was collected and mixed with silica gel to pass through a column for purification, with a yield of 75%.
[0245] 2) Synthesis of intermediate M2-4: under nitrogen atmosphere, (29.1g, 60mmol) compound M2-3, (15.2g, 60mmol) biboronic acid pinacol ester, (11.8g, 120mmol) potassium acetate, (2.6 g, 3.6mmol) Pd(ppf)C...
Embodiment 3
[0248] The synthesis of embodiment 3 compound (M3)
[0249] synthetic route:
[0250]
[0251] 1) Synthesis of intermediate M3-3: according to the synthesis method of compound M2, compounds M3-1 and M3-2 were substituted for compounds M2-4 and M2-6, and the yield was 75%.
[0252] 2) Synthesis of intermediate M3-4: under nitrogen atmosphere, (24.8g, 60mmol) of compound M3-3 and 150mL of triethyl phosphite were added into a 250mL three-neck flask, heated at 160°C, and reacted for 12 hours. The reaction was stopped, and the liquid in the reaction solution was distilled out with a vacuum distillation device. The remaining solid was recrystallized with dichloromethane and ethanol solution, and the yield was about 80%.
[0253] 3) Synthesis of intermediate M3-6: According to the synthesis method of compound M1-3, compound M3-5 (19.8 g, 100 mmol) was substituted for compound M1-1, and the yield was 75%.
[0254] 4) Synthesis of compound M3: according to the synthesis method of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com