Production process for cationic polyester melt and application method for cationic polyester melt
A cationic polyester and production process technology, applied in the field of fiber processing, can solve the problems of volatile organic compounds affecting the environment, inability to use cationic polyester fibers, and increasing production safety hazards.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
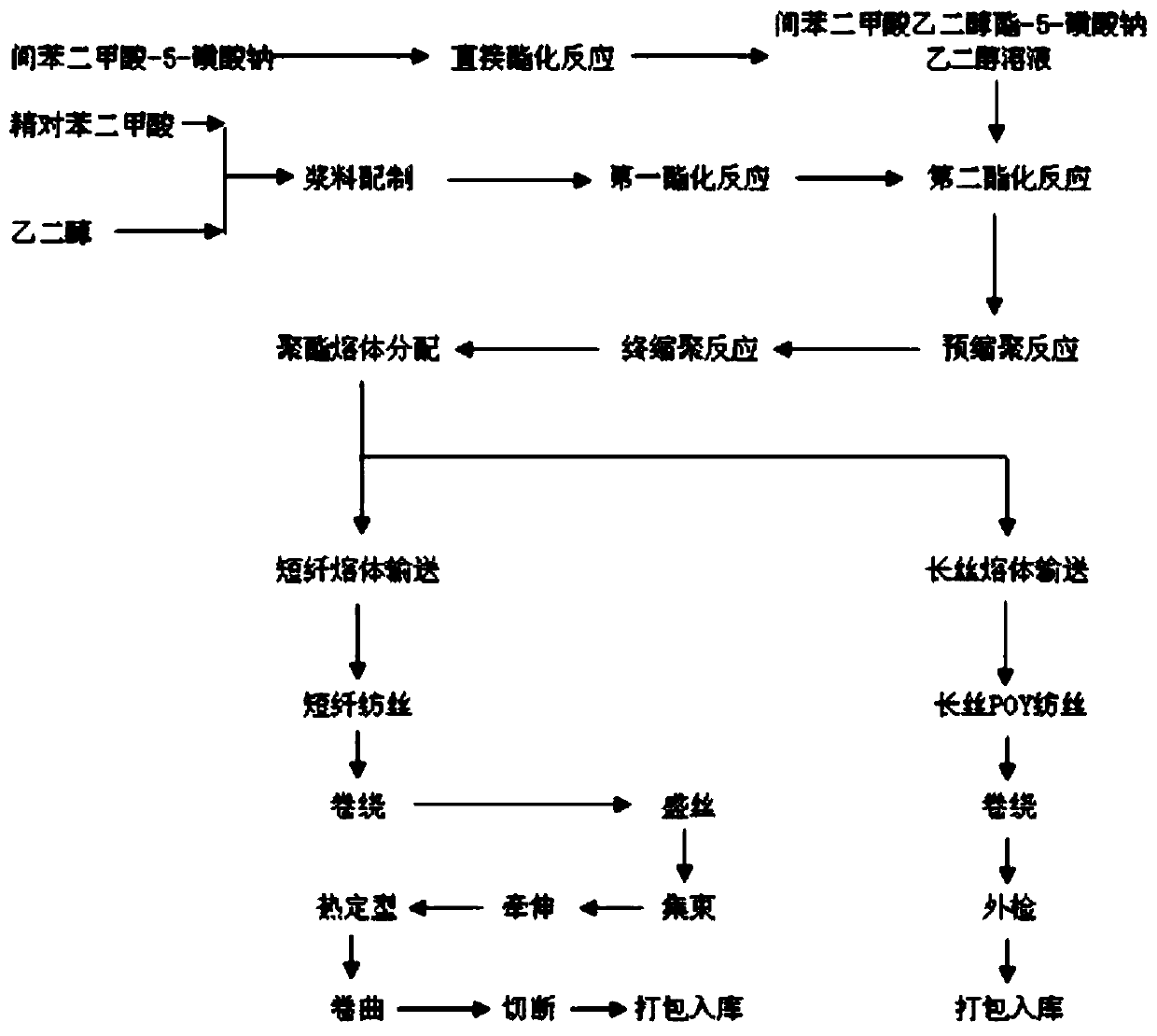
[0041] Embodiment 1 (production of cationic polyester melt)
[0042] (1) With isophthalic acid-5-sodium sulfonate (SIPA) as raw material, acid ion (H + ) under the catalysis of the composite catalyst produced, carry out the esterification reaction with ethylene glycol in the esterification reactor, under the condition of certain reaction temperature and stirring uniformly, finally obtain the esterified product ethylene glycol isophthalate-5- Sodium sulfonate (SIPEG); the mass of the composite catalyst added is 0.2% of the mass of ethylene glycol isophthalate-5-sodium sulfonate, and the reaction temperature in step (1) is 170-190° C.
[0043] (2) Take purified terephthalic acid as raw material, under the effect of antimony series catalyst, carry out esterification reaction with ethylene glycol in the esterification reactor, under certain reaction temperature, reaction pressure and under the uniform situation of stirring, The esterified product ethylene terephthalate (BHET) is ...
Embodiment 2-6
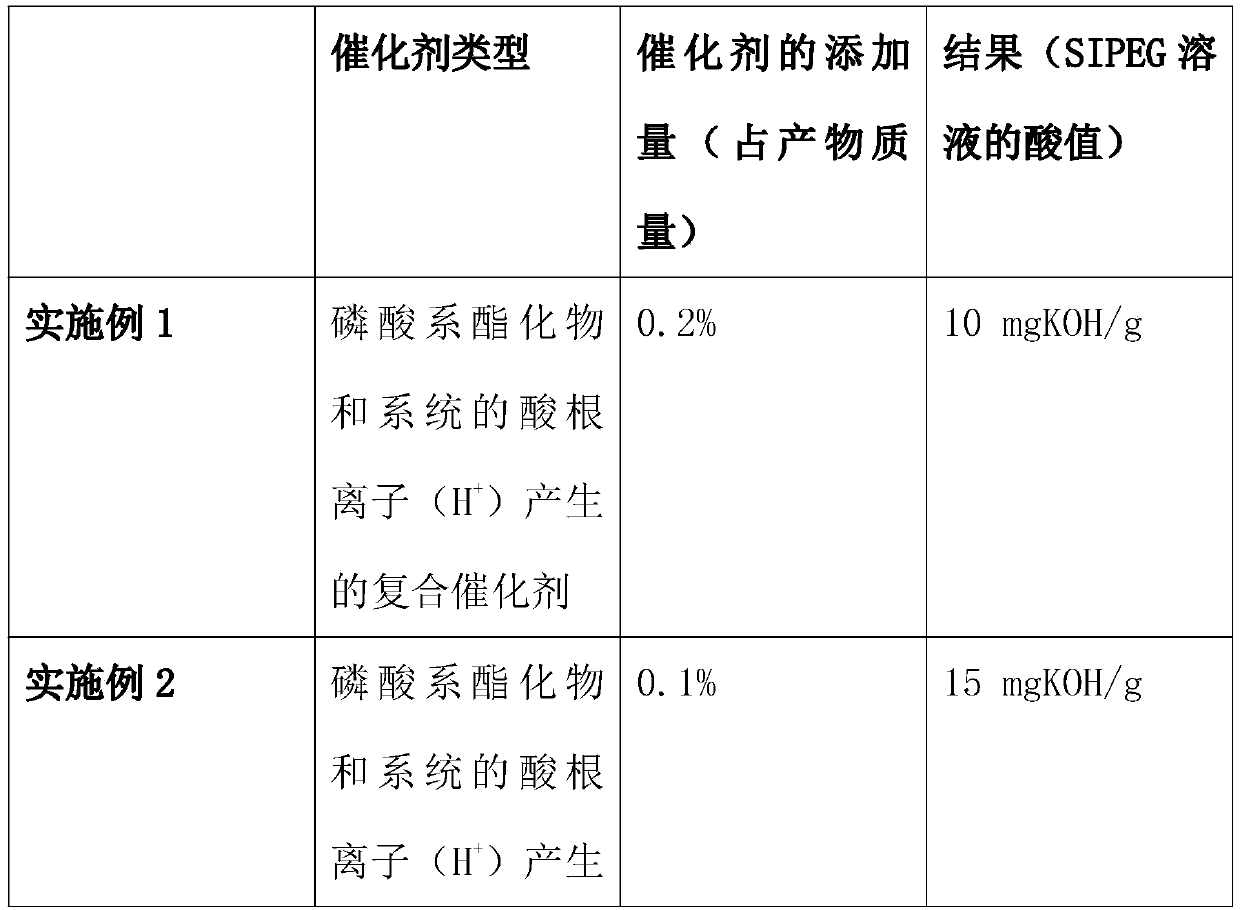
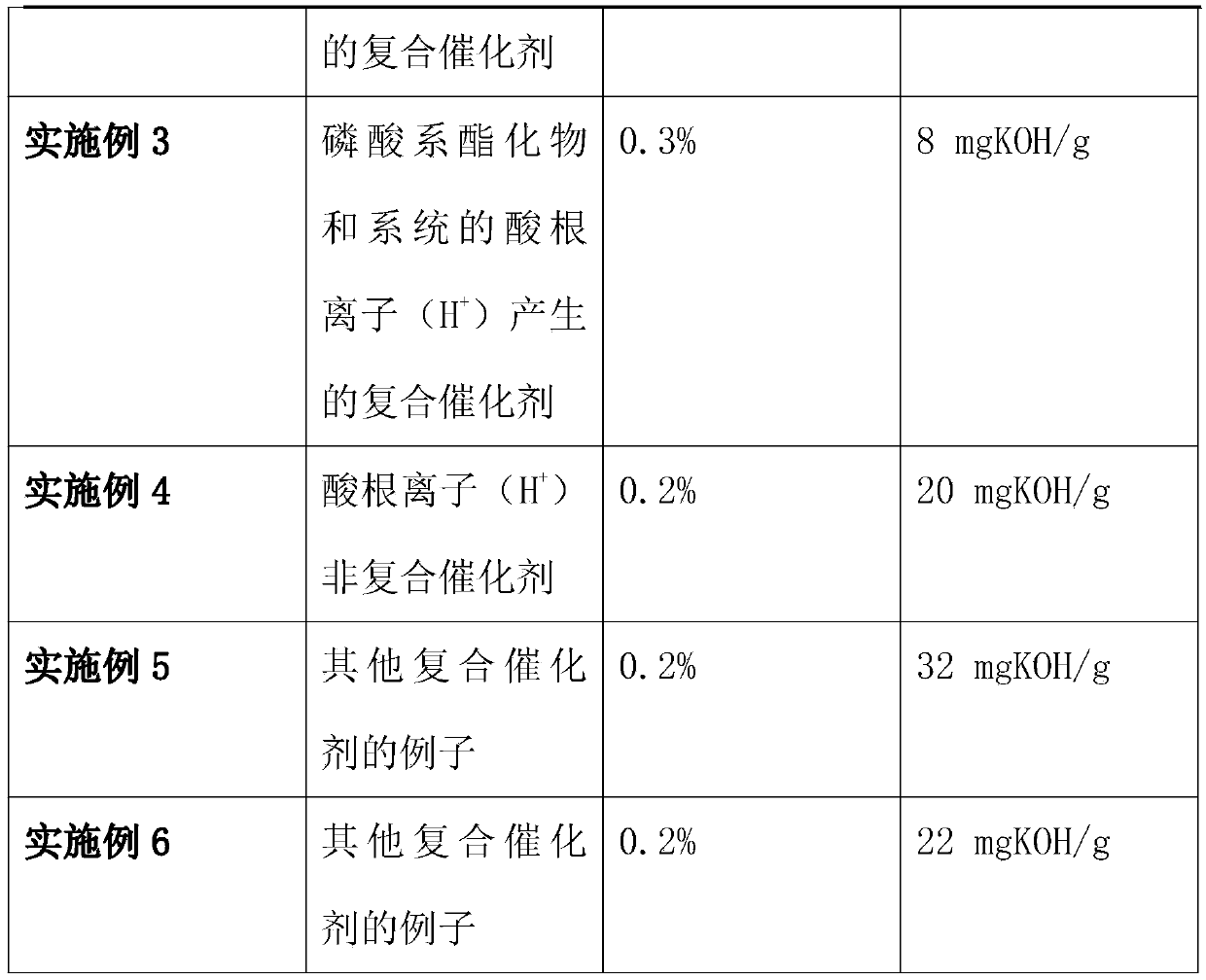
[0049] Others are all the same as in Example 1, except that the catalyst in step 1 or the amount of catalyst added are different, as shown in the following table:
[0050]
[0051]
[0052] For the research on the synthesis of ethylene glycol isophthalate-5-sodium sulfonate, the key is to choose a suitable catalyst, because some side reactions will occur during the chemical reaction, and the reaction time is too long to control the side reactions. From the mechanism analysis of the esterification reaction, the acid radical ion (H + ) played a catalytic role, but the actual process of the reaction will generate a large amount of reaction heat, which is not conducive to the effective control of the esterification reaction, so it is necessary to select some chemical reagents to compound the catalyst to optimize the catalytic effect. After repeated experiments and comparisons, phosphoric acid esters are preferred among phosphates, phosphoric acid esters and antimony compound...
Embodiment 7-10
[0054] Others are all the same as in Example 1, except that the intrinsic viscosity of the prepolymer in step 4 is different, as shown in the following table:
[0055]
[0056] The intrinsic viscosity of the material in the pre-condensation reactor will affect the further chemical reaction of the final polycondensation, because the polymer is also degraded in the process of continuous chain formation, that is, the molecular chain of the polymer compound will be affected by the chemical reaction heat or a very small amount of oxygen. When chemical decomposition occurs, the molecular chain will break, and the chain will continue to form while the broken chain will break. This is a special phenomenon in the process of polymer formation. The degree of decomposition can be determined by detecting the terminal carboxyl group of the material in the prepolymerization reactor. Judgment, the high side of the carboxyl end data indicates that the thermal degradation of the material is o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| Ash content | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com