A new compound and its application
A technology for compounds and compositions, applied in the fields of drug combination, digestive system, biochemical equipment and methods, etc., can solve problems such as hidden safety hazards, incompatibility, accumulation of toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
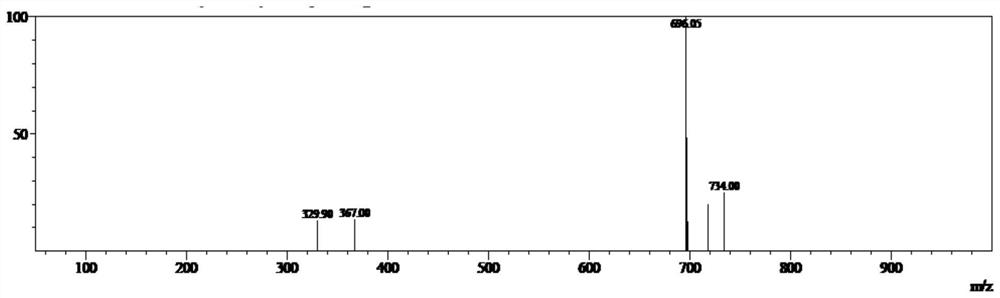
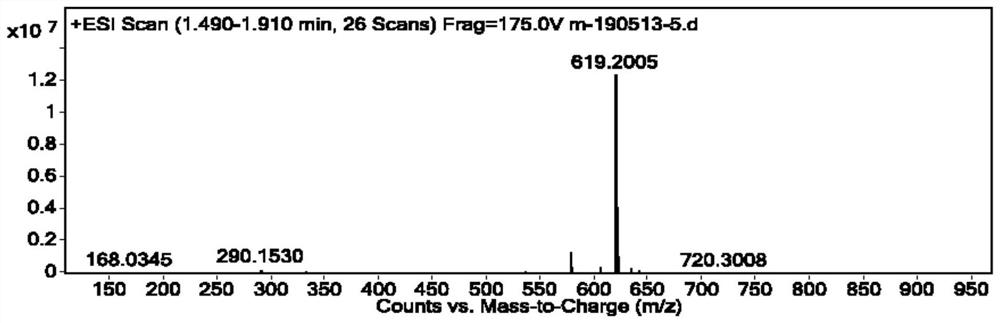
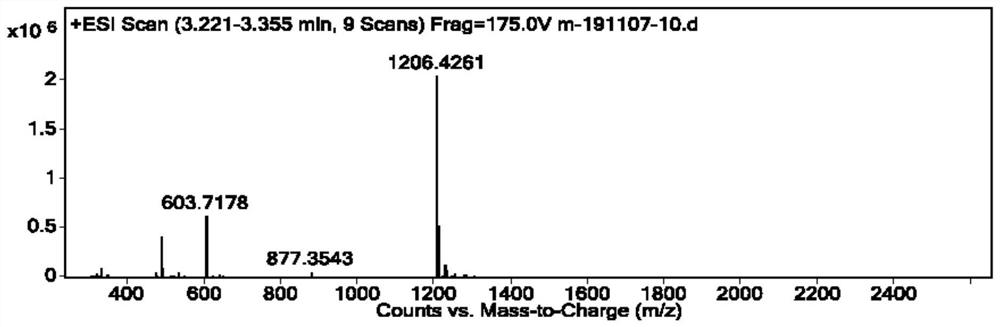
Image
Examples
Embodiment 1
[0146] Embodiment 1, the synthesis of GBL-0401
[0147] 1. Synthesis of Kys-01
[0148] 1.1. Compounds with 5'YICd-01 structure: Synthesis of 5'YICd-01-PFP
[0149] 1.1.1. Synthesis of 5'YICd-01-c1
[0150]
[0151] Measure 5.0 g (81.9 mmol) of 2-hydroxyethylamine, add 50 mL of dimethyl sulfoxide, 5 mL of sodium hydroxide solution (concentration 1 g / mL), add 12 mL (81.9 mmol) of tert-butyl acrylate dropwise for 1 hour, and After reacting for 24 hours, 100 mL of petroleum ether was added, washed twice with saturated brine, and the organic layer was dried. After passing through the column, 7.5 g of a colorless oily substance was obtained.
[0152] 1.1.2. Synthesis of 5'YICd-01-c2
[0153]
[0154] Weigh 7.5g (39.7mmol) of 5'YICd-01-c1, add 50mL of DCM, 23mL of sodium carbonate solution (25%), add 7.7g (45.0mmol) of benzyl chloroformate dropwise at room temperature, react overnight at room temperature, wash with saturated saline Twice, dried over anhydrous sodium sulfate...
Embodiment 2
[0212] Embodiment two, the synthesis of GBL-0402
[0213] 1. Synthesis of Kys-02
[0214] 1.1. Compounds with 5'YICc-01 structure: Synthesis of 5'YICc-01-PFP
[0215] 1.1.1. Synthesis of 5’YICc-01-c1
[0216]
[0217] Take 7.0g (40.0mmol) of SANC-c8 and 9.2g (34.4mmol) of 5’YICd-01-c3, add 25mL of DMF to dissolve, add 9.0g of TBTU, cool down to 10°C, add 2ml of DIEA, and react overnight at room temperature. Add 30 mL of water and 50 mL of dichloromethane, and wash the organic layer three times with saturated brine. The organic layer was dried and evaporated to dryness under reduced pressure. After passing through a column chromatography (eluent: dichloromethane: methanol = 1%-10%), 10.0 g of a yellow viscous solid was obtained.
[0218] 1.1.2. Synthesis of 5'YICc-01-c2
[0219]
[0220] Take 10.0g of 5'YICc-01-c1, add 15ml of concentrated hydrochloric acid, and react overnight at room temperature. Evaporate to dryness under reduced pressure to obtain 7.3g.
[0221...
Embodiment 3
[0251] Embodiment three, the synthesis of GBL-0403
[0252] 1. Synthesis of Kys-03
[0253]1.1. Compounds with 5’ERCd-01 structure: Synthesis of 5’ERCd-01-PFP
[0254] 1.1.1. Synthesis of 5’ERCd-01-c1
[0255]
[0256] Measure 5.0g (54.9mmol) of 2-amino-1,3-propanediol, add 50mL of DMSO, 5mL of sodium hydroxide solution (concentration: 1g / mL), cool down to 0°C, add dropwise 20mL (137.8mol) of tert-butyl acrylate2 After the addition was completed in 1 hour, react at room temperature for 48 hours, add 100 mL of petroleum ether, wash with saturated brine twice, and dry the organic layer. After passing through a chromatography column (eluent: ethyl acetate: petroleum ether = 25%-75%), add 0.05% triethylamine to the column to obtain 6.2 g of a colorless oil.
[0257] 1.1.2. Synthesis of 5’ERCd-01-c2
[0258]
[0259] Weigh 6.2g (17.9mmol) of 5'ERCd-01-c1, add 50mL of dichloromethane, 23mL of sodium carbonate solution (25%), add 8.2mL of benzyl chloroformate (57.4mmol) dro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com