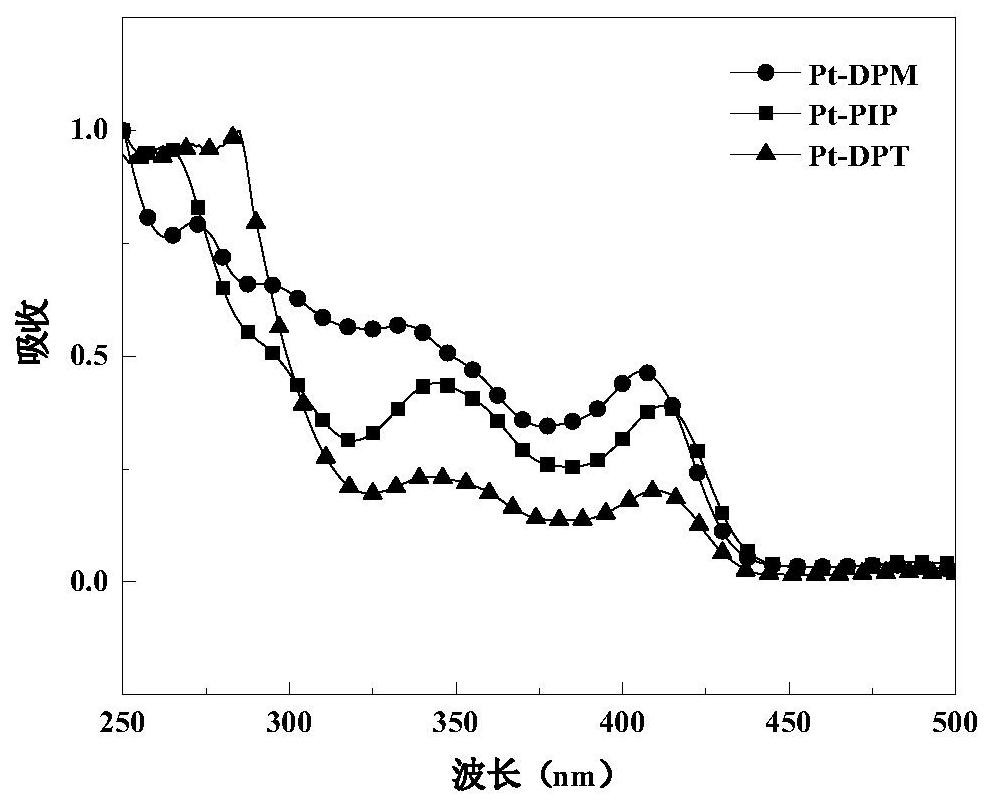
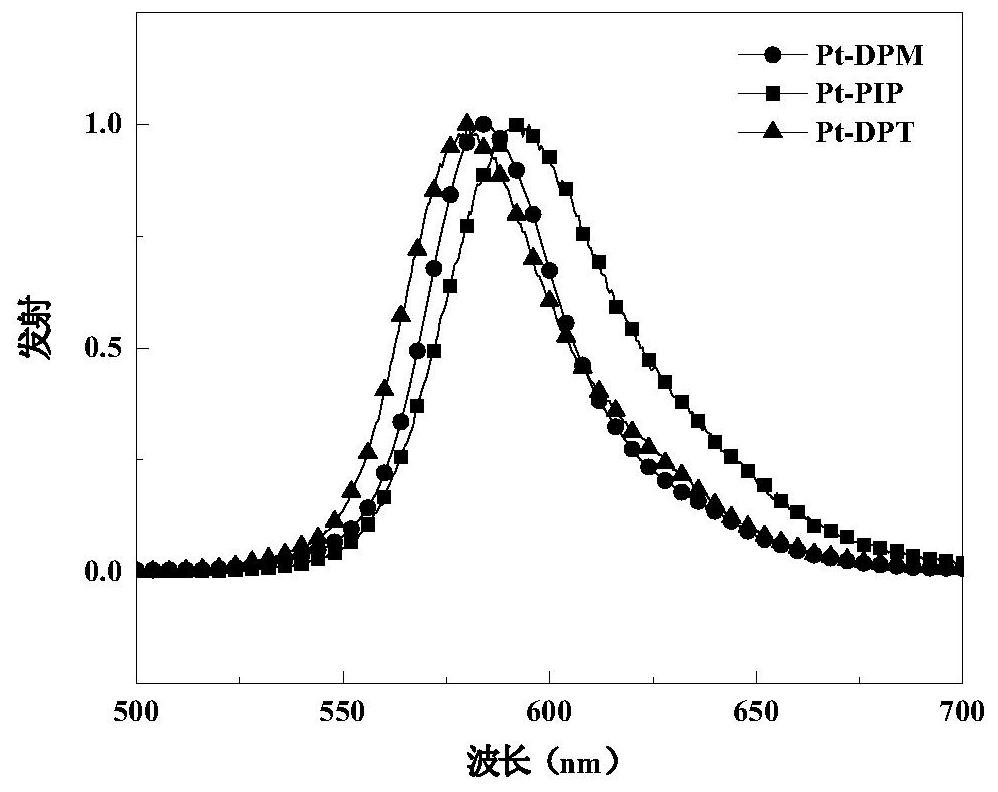
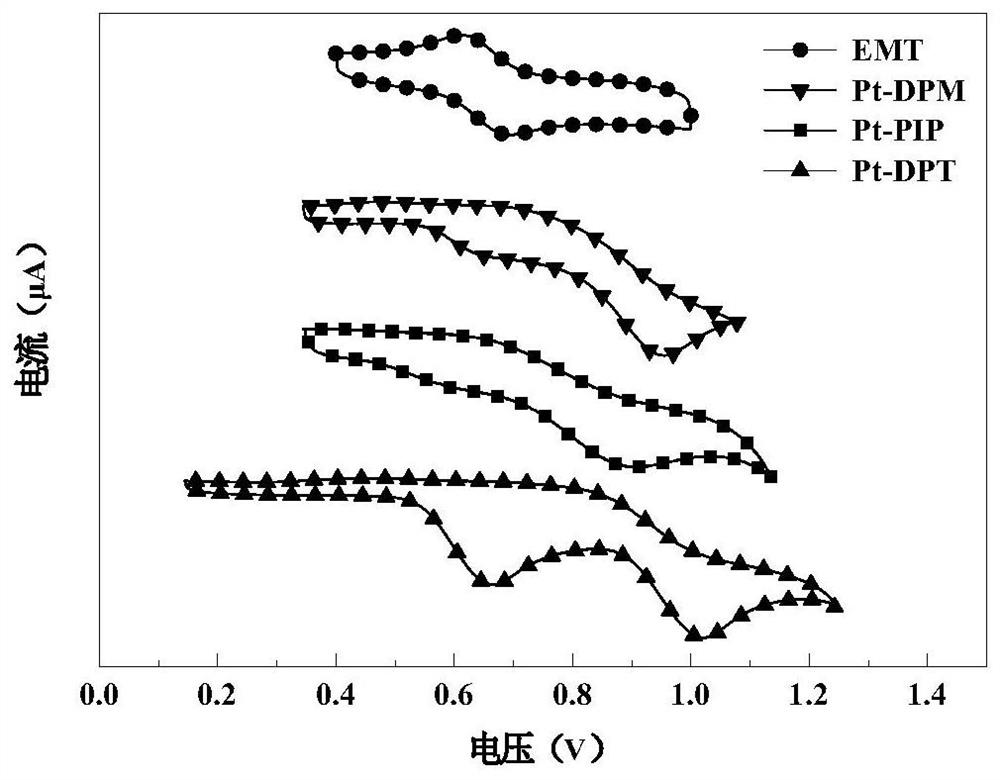
Pterene pyridazine tetradentate platinum complex phosphorescent material and its preparation method and application
A technology of platinum complexes and phosphorescent materials, applied in luminescent materials, platinum-based organic compounds, platinum-group organic compounds, etc., can solve the problems of limiting wide application and limited stability of OLEDs, and achieve improved color purity and reduced non-radiative transitions , Inhibit the effect of π-π stacking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of Intermediate 9,10-Dihydro-9,10-Diethyl Antholic-11,12-Acid Anhydride DDA
[0049] The anthracene (7.5 g, 42 mmol) and butyridate (DMAD, 7.5 mL, 61 mmol) were placed in the reaction bottle, reacted at 170 ° C for 45min, then warmed to 180 ° C for 5 min. After the reaction was completed, the mixture was cooled, recrystallized with methanol, and 12.2 g of a white solid 9,10-dihydro-9,10-diethylntylene-11, 12-dicarboxylate DDCME was obtained, and the yield was 90%. The above-mentioned product DDCME was dissolved in sodium hydroxide (4.0 g, 100 mmol), methanol (50 mL, 1233 mmol), water (15 mL, 833 mmol), reflux for 1 hour, and then placed at -20 ° C overnight. There is crystal, dissolved in water, adjusting pH to 5 or less with 1 mole / liter dilute hydrochloric acid solution, precipitated, filtrate dried to give 10 g of white solid 9,10-dihydro-9,10-diethyl anthantant 11, 12-dicarboxylic acid DDCA, yield is 89%. DDCA (10 g, 34 mmol) and sodium acetate (...
Embodiment 2
[0051] Example 2, Preparation of DDCP DDAH and Dichlorozine Compound DDCP
[0052] Taking 9,10-dihydro-9,10-diethylhrane-11, 12-acid anhydride DDA (6.5 g, 23 mmol) to the reaction bottle, poured (20 mL, 350 mmol) glacial acetic acid, stirred back. After the solid dissolution, hydrazine (3.5 g, 70 mmol) was added dropwise, and the reaction was reacted at 125 ° C for 3 h. Cooling, filtration, washed with ethanol, dried to give 6 g of a butterofene modified intermediate hydrazide DDAH, yield 90%. DDAH (6.0 g, 21 mmol) was placed in the reaction bottle, evacuated, and the filled charge was repeatedly replaced, 1,2-dichloroethane (20 mL, 249 mmol) was added, and trichlorochlorophos (16 ml, 172 mmol) was added. The reaction was reacted at 125 ° C for 8 h. Vacuum ol drying 1,2-dichloroethane and trichlorophosphorus, poured the product into ice water, adjusted pH to neutral, precipitated with sodium hydroxide solution, precipitated solid, filtered, and dichloromethane obtained Dissolve, d...
Embodiment 3
[0054] Example 3, Preparation of 2,6-dimethylphenoxazine Iddp in ligand butteritis modified
[0055] Popular dichlorozine DDCP (1.71 g, 5 mmol), 2,6-dimethylphenol (0.49 g, 4 mmol), potassium carbonate (1.38g, 10 mmol), add the reaction bottle, vacuum, charge Nitrogen was repeatedly replaced at least three times; DMF after 10 ml of anthracene gas was added, and stirred at 120 ° C for 24 h. After completion of the reaction, extract with dichloromethane, dried over anhydrous sodium sulfate, filtration, column chromatography, with V PE : V EA = 5: 1 Anchor chromatography was separated to give a white solid 1.38 g, and the yield was 84%. 1 H NMR (400 MHz, DMSO) δ = 7.73-7.60 (m, 4H), 7.19-7.08 (m, 7H), 6.32 (S, 1H), 6.10 (S, 1H), 1.97 (S, 6H).
[0056]
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com