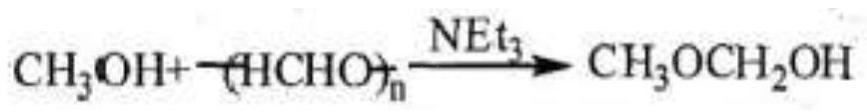A process for catalyzing synthesis of glyphosate
A glyphosate and process technology, applied in the field of catalytic synthesis of glyphosate by glycine method, can solve the problems of increasing mother liquor production, increasing environmental protection pressure, and affecting economic benefits, achieving quality advantages, small tail gas, and depolymerization The effect of complete response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
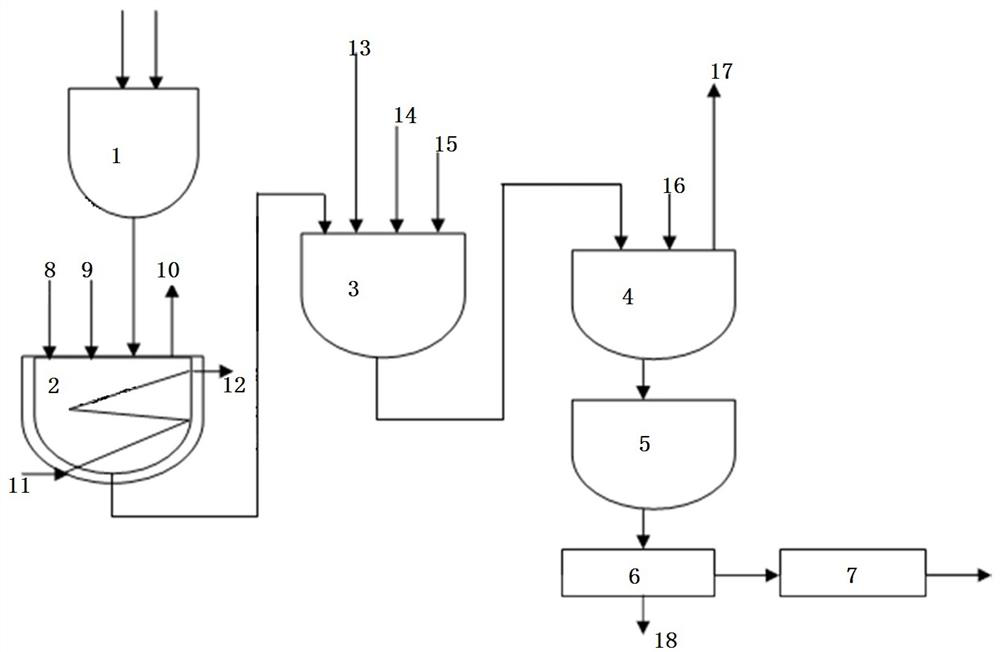
Image
Examples
Embodiment 1
[0044]1. Depolymerization reaction: First, put paraformaldehyde into the depolymerization kettle (the degree of polymerization of paraformaldehyde is 38), open the valve of the depolymerization kettle connected to the exhaust system pipeline, pass steam through the jacket, and heat and dehydrate at 60 ° C for 2 minutes . After dehydration, add methanol (the molar ratio of methanol and paraformaldehyde is controlled at 0.5:1), stir evenly, and add methanol solution of sodium methoxide to the mixed solution, and the depolymerization can be quickly completed in 30 seconds at room temperature. The methanol solution of active anhydrous formaldehyde, after the depolymerization is completed, add methanol until the molar ratio of paraformaldehyde to alcohol is 1:7.
[0045] The molar ratio of sodium methoxide to formaldehyde (the number of moles of paraformaldehyde is calculated as formaldehyde) is controlled: five parts per million.
[0046] 2. Addition reaction: After the depolymer...
Embodiment 2
[0052] 1. Depolymerization reaction:
[0053] First put paraformaldehyde into the depolymerization kettle (the degree of polymerization of paraformaldehyde is 50), open the valve of the pipe connected to the tail gas system of the depolymerization kettle, pass steam through the jacket, heat and dehydrate at 60 ° C for 3 minutes, and then add it after the dehydration is completed. Methanol (the molar ratio of methanol and paraformaldehyde is controlled at 0.6:1), stir evenly, add potassium methoxide solid to the mixed solution, and naturally react at 30 ° C for 20 s for depolymerization to generate highly active anhydrous formaldehyde. methanol solution; after the depolymerization is completed, add ethanol until the molar ratio of paraformaldehyde to alcohol is 1:7.8.
[0054] Molar ratio control of lithium methoxide and formaldehyde (the number of moles of paraformaldehyde is calculated as formaldehyde): 2 parts per million.
[0055] 2. Addition reaction:
[0056] After the ...
Embodiment 3
[0062] 1. Depolymerization reaction: The purchased alcohol solution of anhydrous formaldehyde is used as the raw material of formaldehyde, the methanol solution of catalyst potassium methoxide is added to the alcohol solution of anhydrous formaldehyde, and the reaction is carried out for 20 seconds; after the depolymerization is completed, methanol is added to paraformaldehyde and alcohol. The molar ratio is 1:2.2;
[0063] The molar ratio of the potassium methoxide to formaldehyde is controlled: five parts per million.
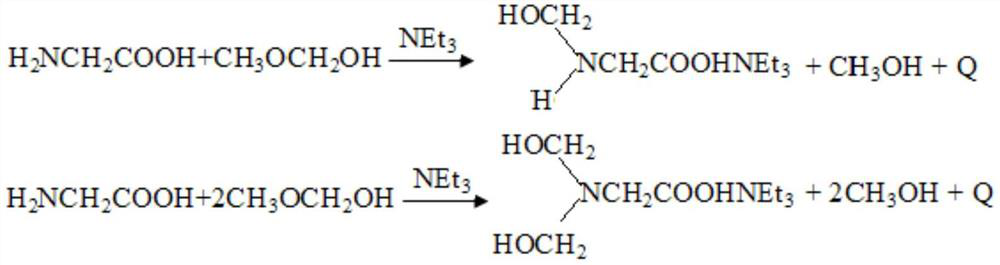
[0064] 2. Addition reaction: After the depolymerization is completed, add triethylamine to the mixed solution, then add glycine, and stir evenly. The addition reaction occurs under the action of the new alkoxy group and the molecules of formaldehyde and hemiacetal. After the addition reaction was completed, the mixed solution was rapidly heated to 60° C., maintained at a temperature of 18 s, then cooled to about 38° C., and then dimethyl phosphite was added ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com