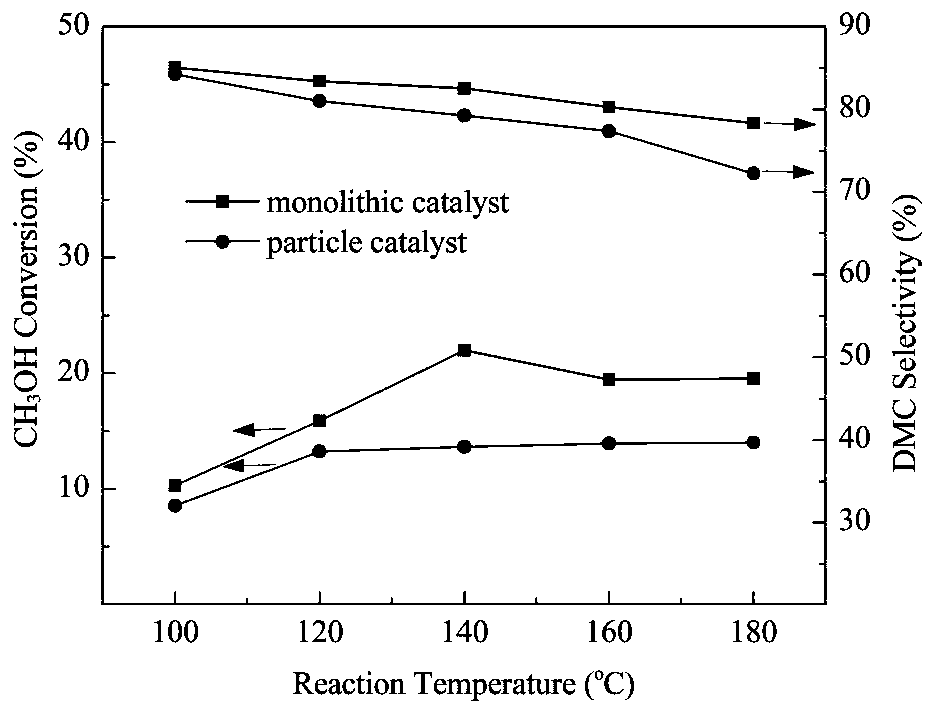
Monolithic catalyst for preparation of dimethyl carbonate by direct synthesis process, preparation method and direct synthesis method of dimethyl carbonate
A monolithic catalyst, dimethyl carbonate technology, applied in the direction of chemical instruments and methods, heterogeneous catalyst chemical elements, physical/chemical process catalysts, etc., to achieve process strengthening, improved catalytic performance, excellent catalytic performance and stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
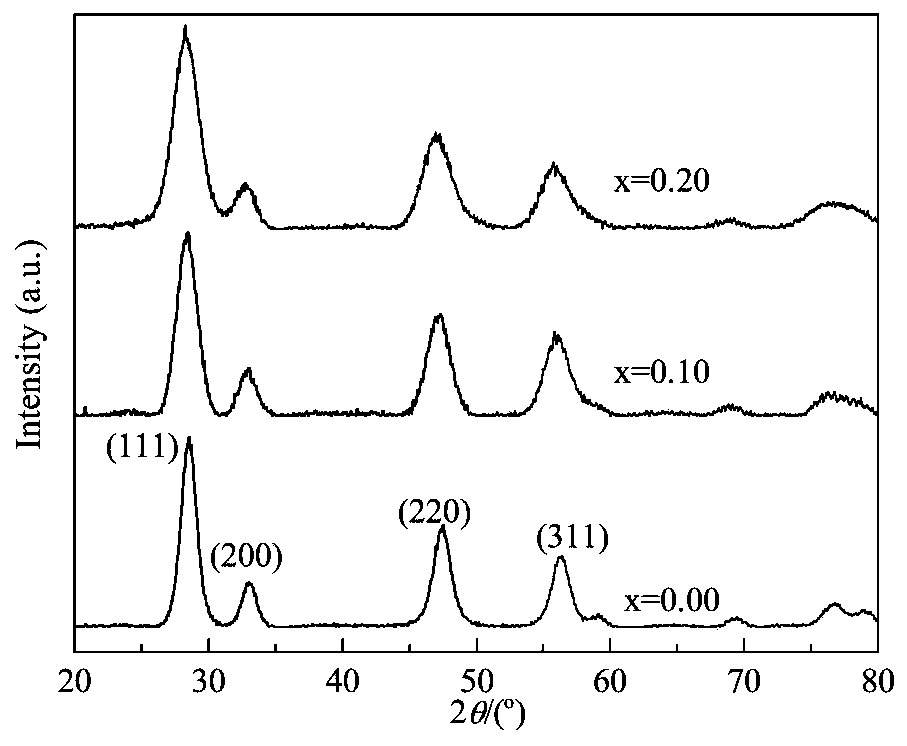
[0024] Preparation of Ce with Lanthanum Oxide Doping 5% 0.95 La 0.05 o δ -Al 2 o 3 Monolithic catalyst (molar ratio of ceria to lanthanum oxide is 0.95:0.05). Methods as below:
[0025] Weigh 15.000g (NH 4 ) 2 Ce(NO 3 ) 6 and 0.624g La(NO 3 )·6H 2 O, stirring and dissolving in an appropriate amount of deionized water to obtain a nitrate precursor mixed solution, stirring and dissolving 70g of urea in an appropriate amount of deionized water, the total amount of deionized water used in the two dissolution processes is 500mL. The nitrate precursor mixture and the urea solution were successively transferred to a 1000mL three-necked flask, heated in a water bath to 80-100°C (preferably 90°C) under mechanical stirring conditions, and kept for 4-6h (preferably 5h), until the When the pH reaches 8-9, cool naturally to room temperature after the reaction is complete, filter and wash the reaction product, then mash the obtained filter cake, add deionized water to make an emuls...
Embodiment 2
[0029] Lanthanum oxide doped with 10 wt% Ce 0.90 La 0.10 o δ -Al 2 o 3 The preparation process of the monolithic catalyst (referred to as Cat 2) is the same as that of Example 1, except that the amounts of each precursor and polyethylene glycol are different, and the specific amounts are shown in Table 1.
Embodiment 3
[0031] Lanthanum oxide doped with 15 wt% Ce 0.85 La 0.15 o δ -Al 2 o 3 The preparation process of the monolithic catalyst (referred to as Cat 3) is the same as that of Example 1, except that the amounts of each precursor and polyethylene glycol are different, and the specific amounts are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com