Preparation and application of mesenchymal stem cell secretion factors
A technology of mesenchymal stem cells and secreting factors, which is applied in the application of promoting angiogenesis and skin anti-aging and repairing, and the preparation of mesenchymal stem cells secreting factors, achieving the effects of high safety, high content, and strong ability to promote skin defect repair.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
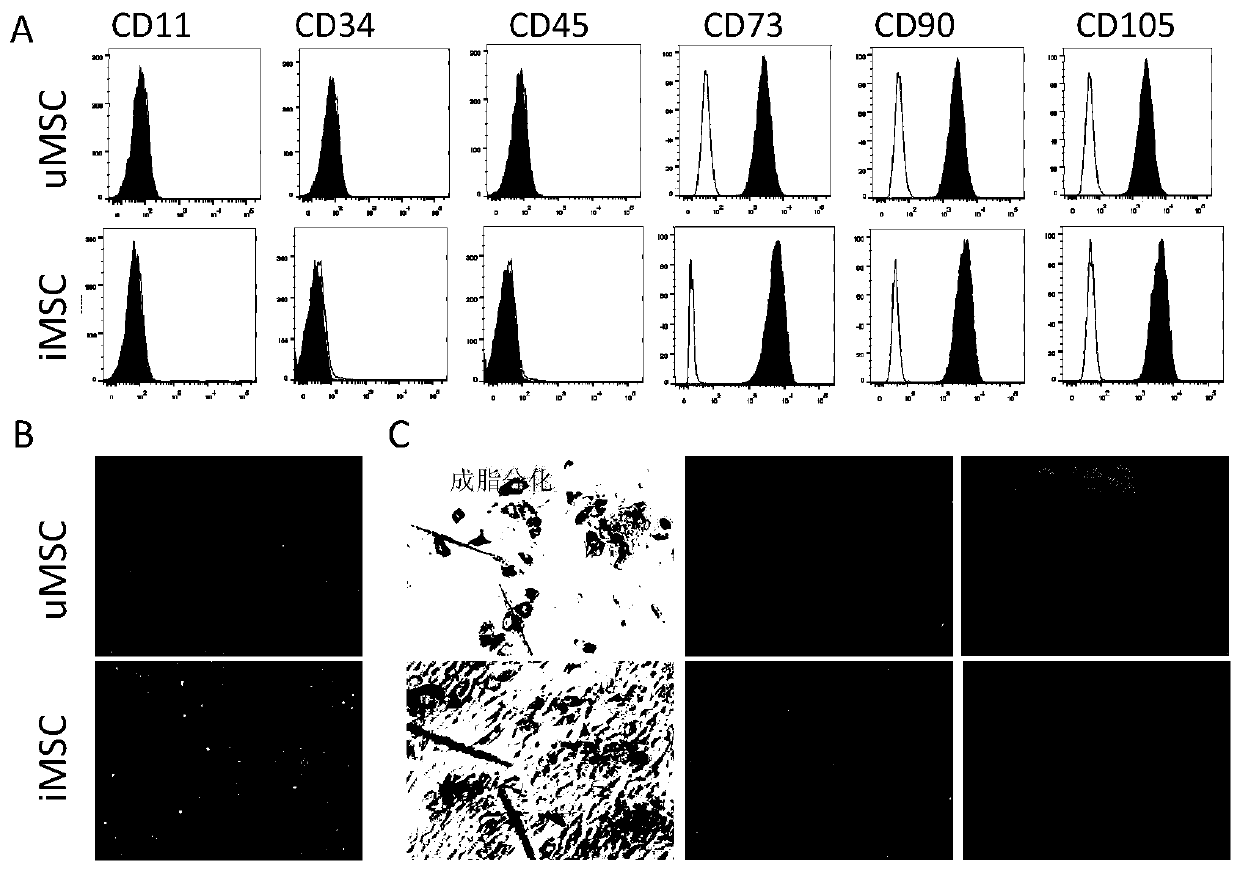
[0045] Example 1 Induction, expansion and identification of iMSCs
[0046] Select iPSCs in good condition, and replace the iPSC medium with complete MSC medium. Medium was changed every 2-3 days for approximately 14 days. Differentiated cells were flow-sorted using the MSC marker CD105 to obtain CD105-positive cells. Use MSC complete medium to continue the expansion culture. MSC complete medium components are: low-sugar DMEM, 10% fetal bovine serum, non-essential amino acids, double antibodies (containing 100units / mL penicillin and 100μg / mL streptomycin), 2mM GlutaMAX, 2ng / mL EGF, 2ng / mLFGF, 55 μM β-mercaptoethanol. Differentiation process see figure 1 .
[0047] After digestion of P4 generation cells with a confluence of 90% (use Tryple E for cell digestion), count and prepare a density of 1 × 10 6 Each cell / mL cell suspension was filtered with a 40 μm cell mesh, 1 mL of the filtrate was taken, and 10 μL of fluorescently labeled cell surface marker antibodies were added...
Embodiment 2
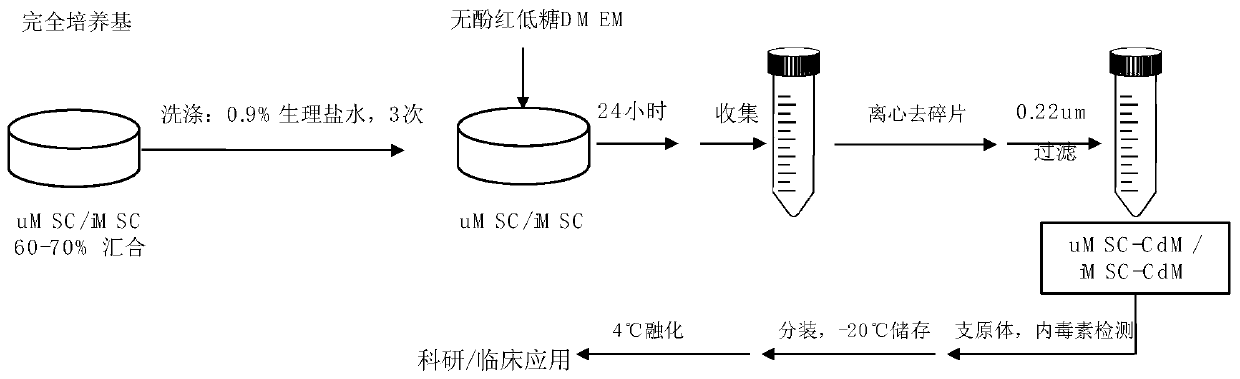
[0051] Example 2 Preparation and storage of iMSC-CdM
[0052] Select P5-P8 generations of iMSCs with strong proliferation ability to prepare the cytokine supernatant; the preparation steps of iMSC-CdM are as follows: P5-P8 generations of iMSCs, when the cell confluence is 60%-70%, aspirate to completely culture After rinsing three times with 0.9% normal saline, add an appropriate amount of phenol red-free low-sugar DMEM (Gibco, #11054) according to the area of the culture dish (see Table 2 for details); after culturing for 24 hours, collect the cell culture supernatant and centrifuge at 1200rpm Cell debris and impurities were removed for 5 minutes, filtered with a 0.22 μm filter membrane, and subpackaged; the iMSC-CdM prepared through the above steps were tested for mycoplasma and endotoxin (third-party testing company), and the results were negative.
[0053] The iMSC-CdM prepared by the above steps were aliquoted and stored at -20°C, thawed at 4°C before use, and stored at...
Embodiment 3
[0058] Example 3 Effect of Different Cell Confluency on Total Protein and Key Components of iMSC-CdM
[0059] The preparation of iMSC-CdM was carried out when the cell confluency was 60-70% and 80-90%, respectively, and the preparation steps were as described in Example 2.
[0060] The total protein concentration in iMSC-CdM was determined by BCA method.
[0061] The concentration of key components in iMSC-CdM including angiogenin, bFGF, HGF, and VEGF was determined by protein chip or Elisa method.
[0062] Such as Figure 4As shown in (A, B), the preparation of iMSC-CdM is carried out when the cell confluence is 60%-70%, the total protein content in iMSC-CdM is about 1.2 times of that of 80%-90% confluence, and the angiogenesis The content of production factors, including angiogenin, bFGF, HGF, and VEGF, was higher than that at 80%-90% confluence. A cell confluence of 60%-70% is more conducive to enriching the protein components in iMSC-CdM than 80%-90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com