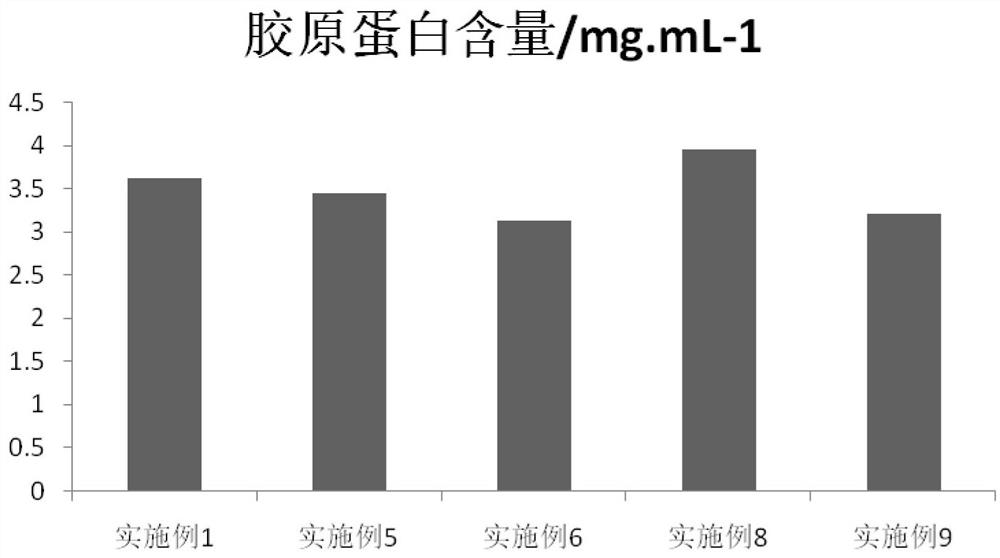
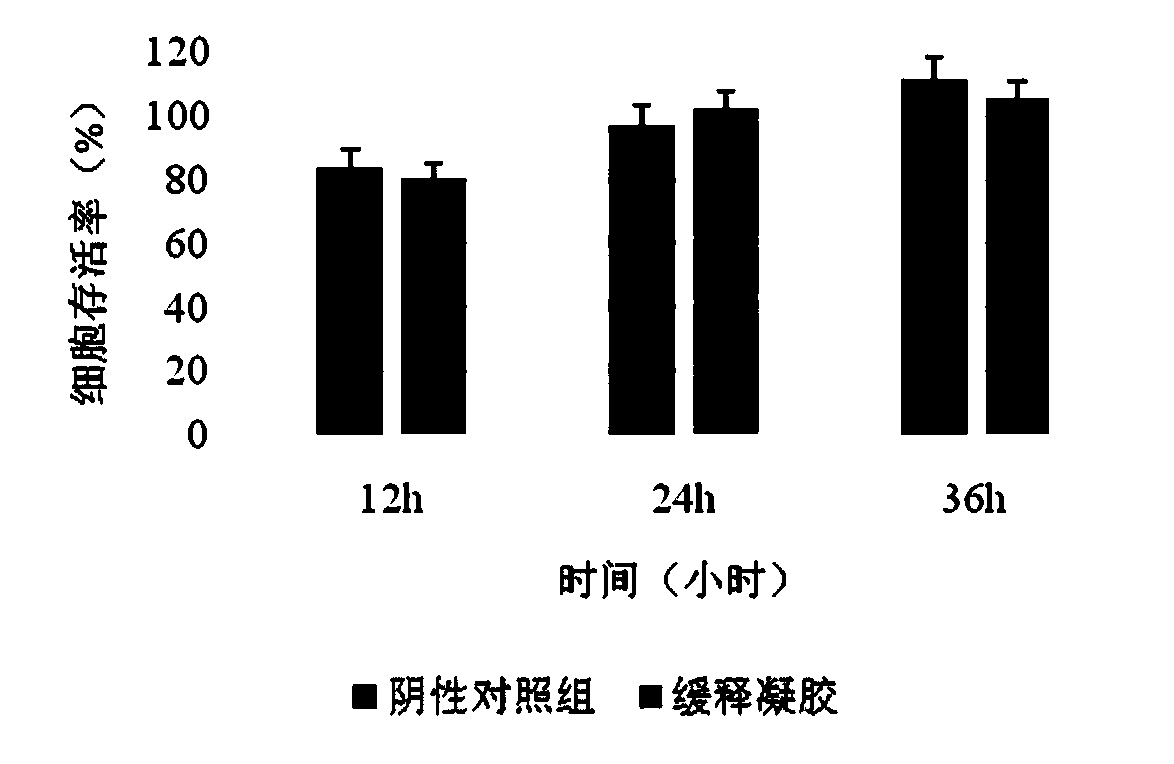
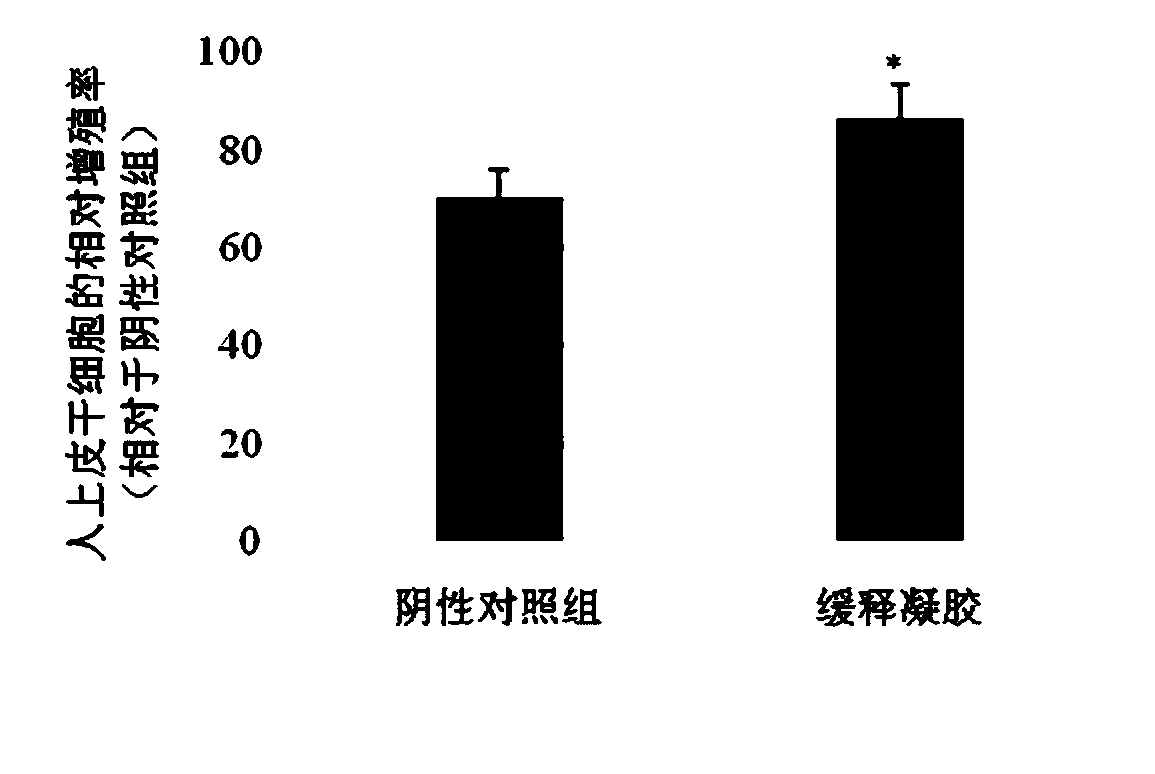
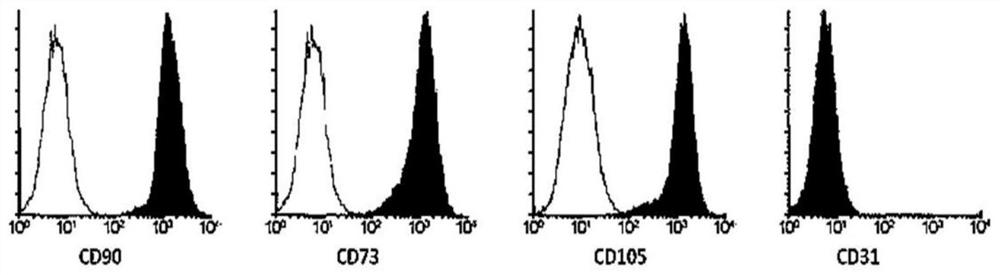
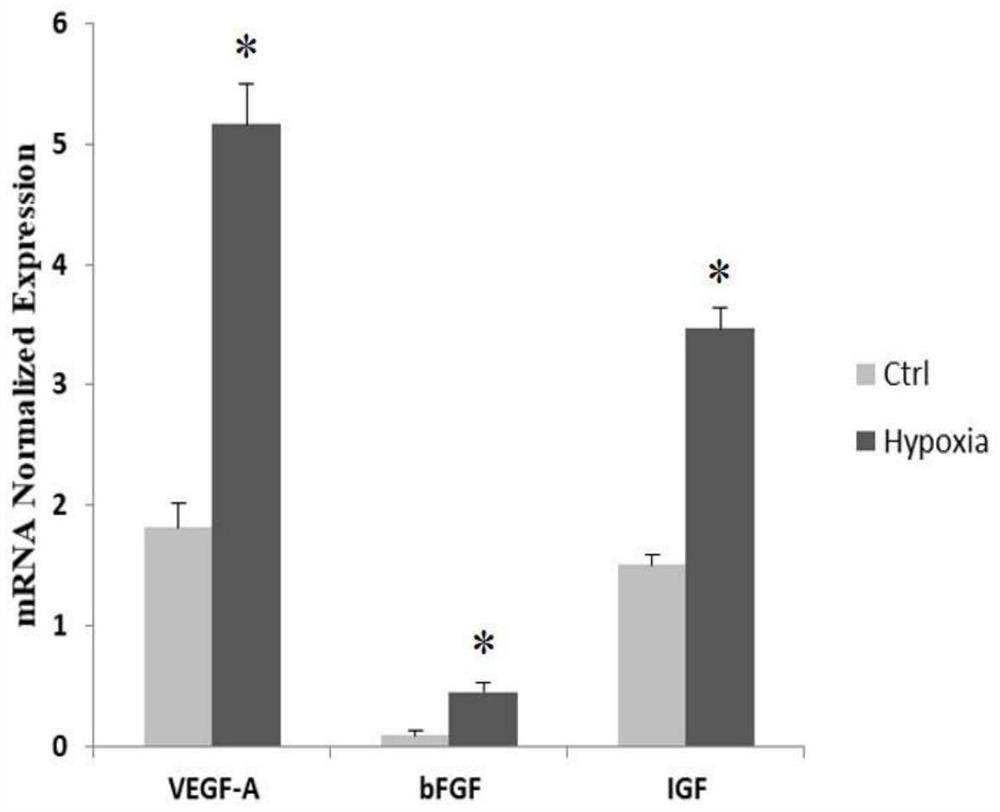
Patents
Literature
39 results about "SECRETOR FACTOR" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
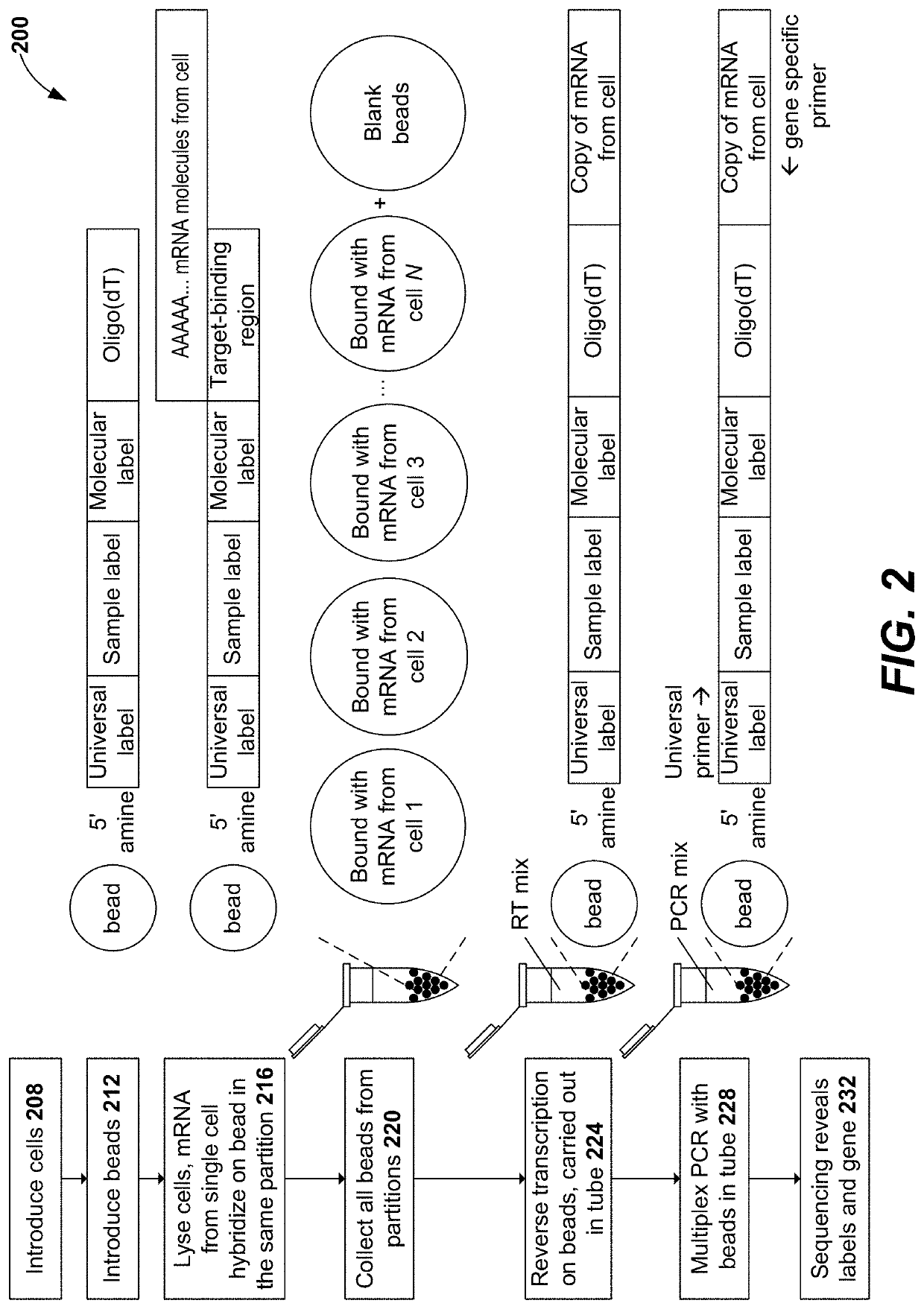
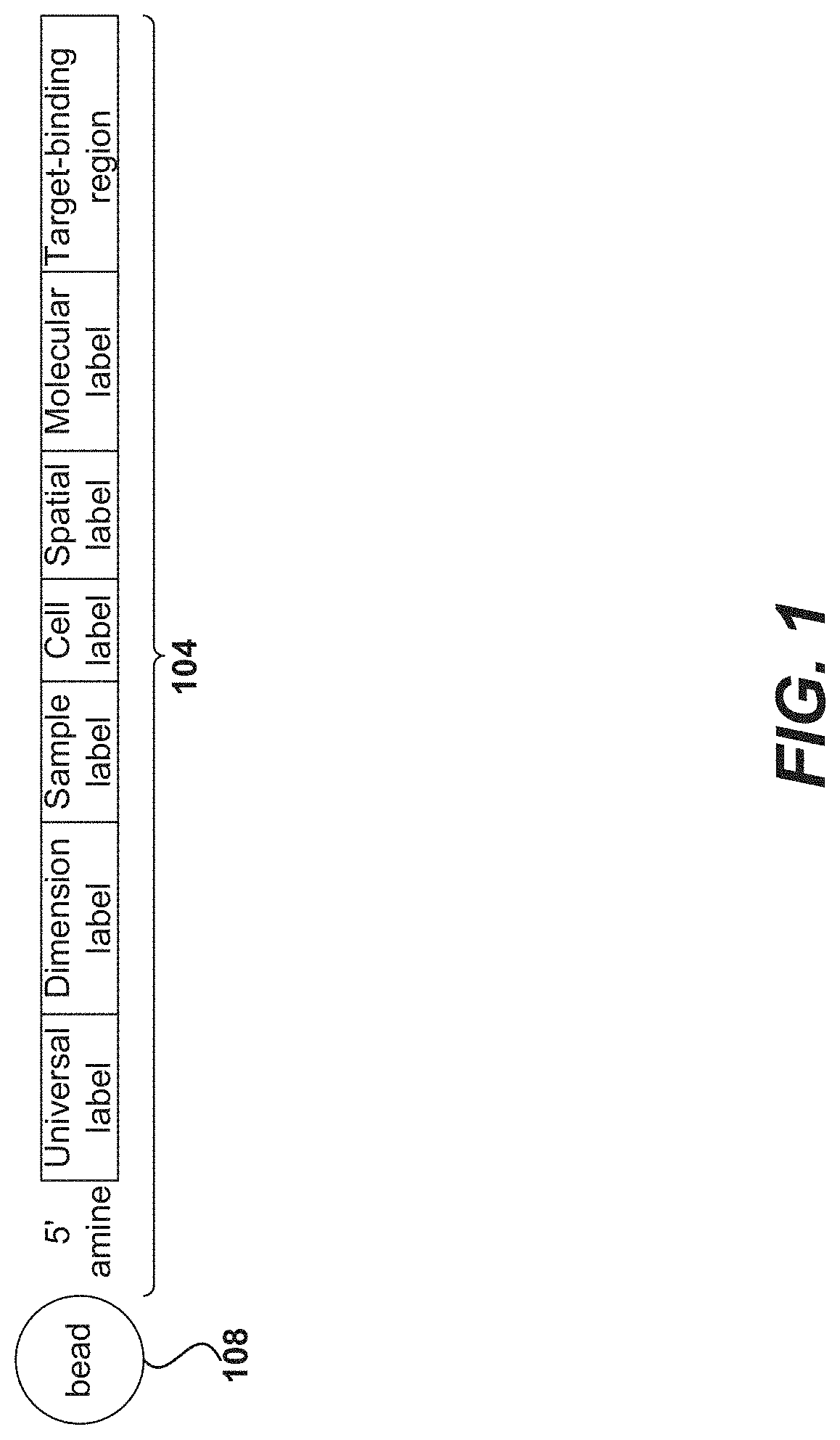
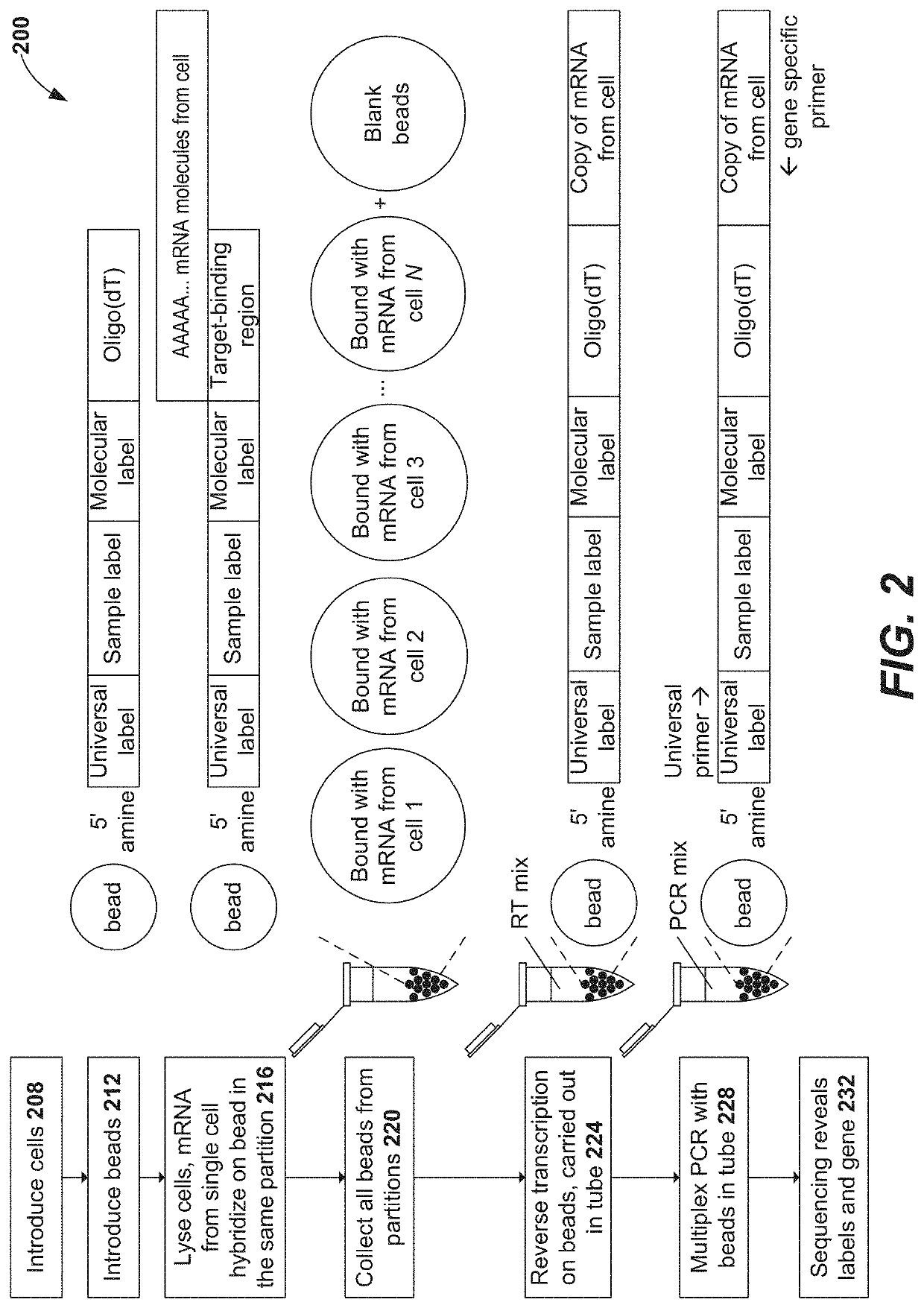
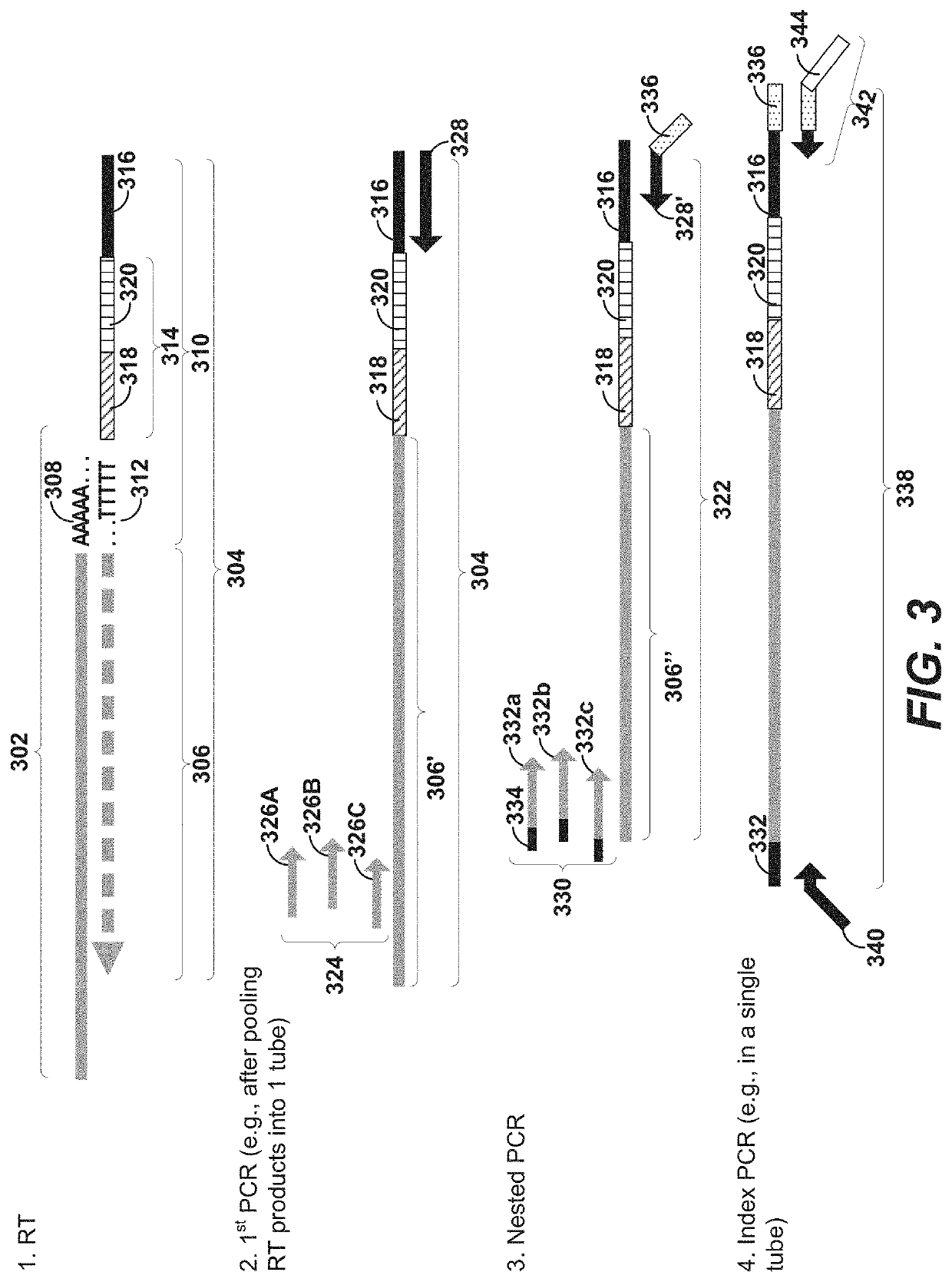
Methods and compositions for single cell secretomics
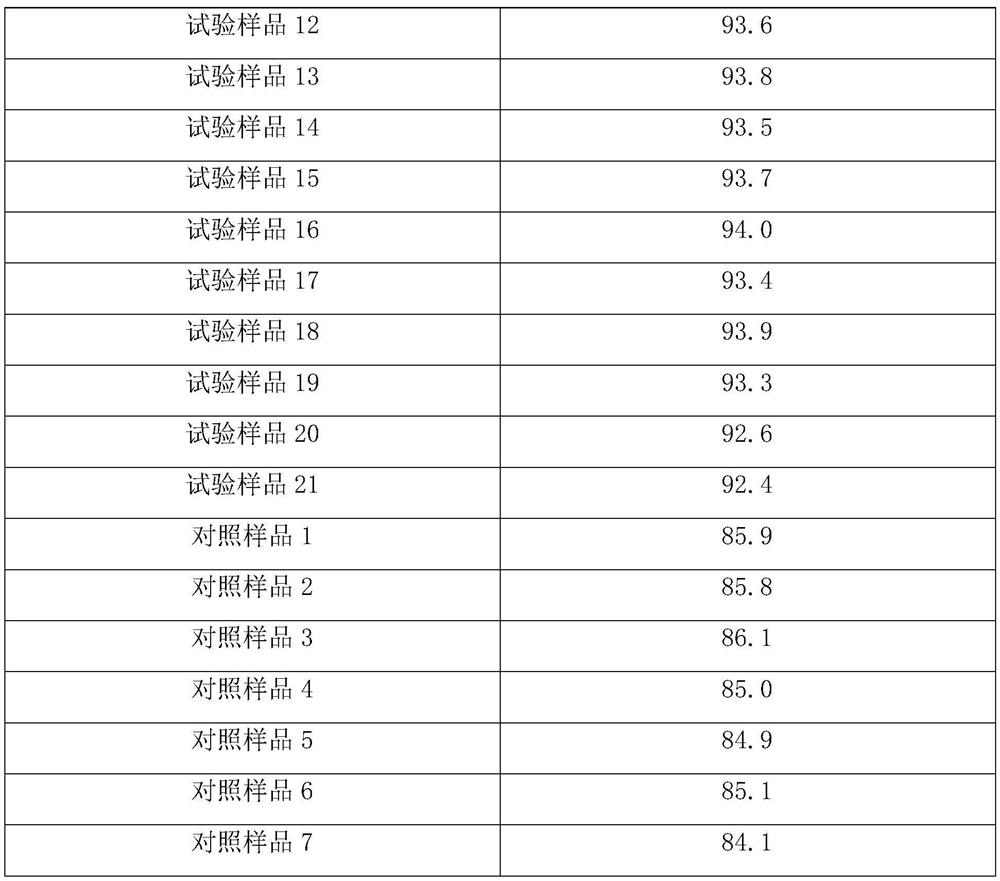
PendingUS20210222244A1Microbiological testing/measurementBiological testingNucleotideCellular secretion
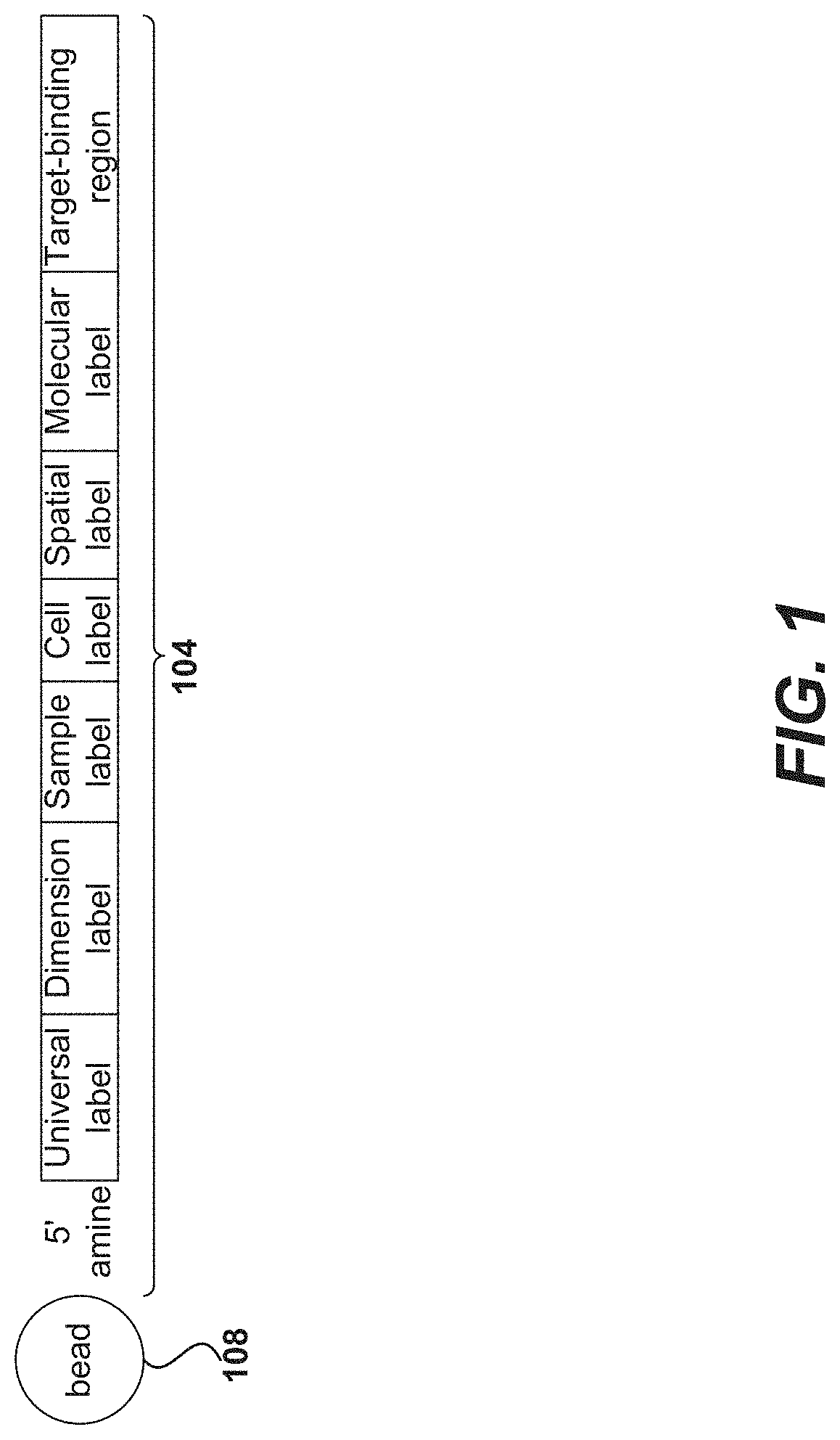
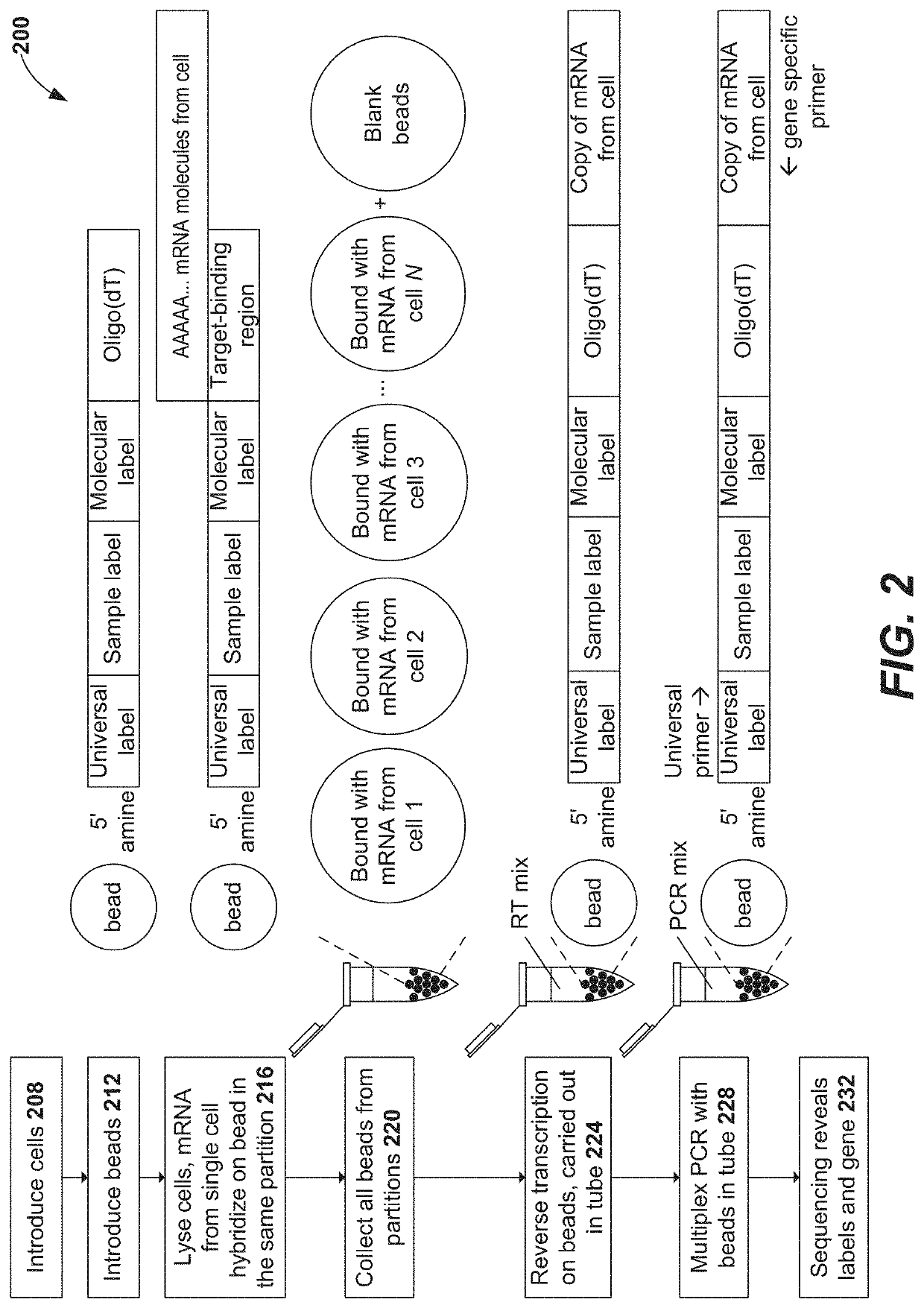
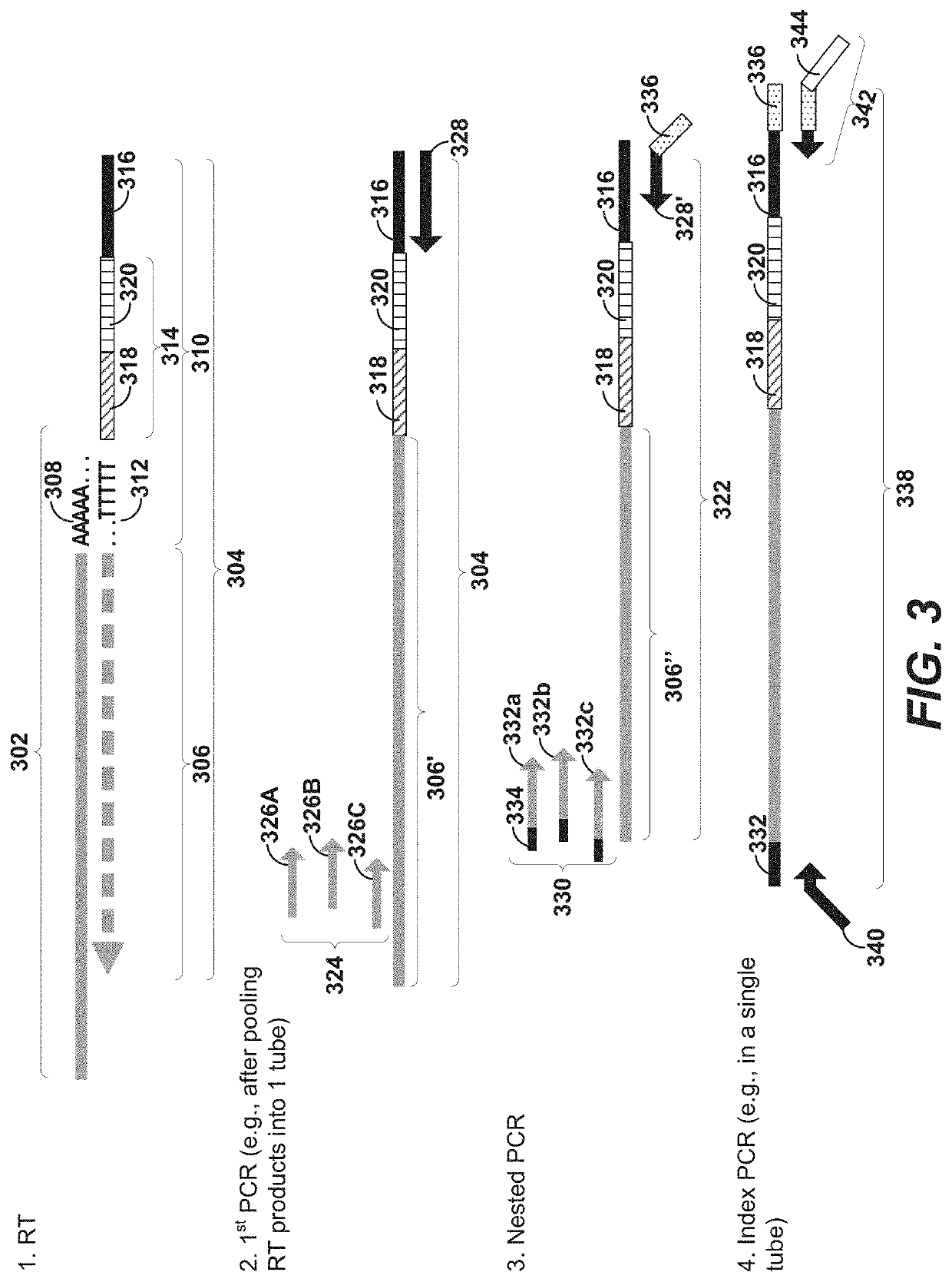
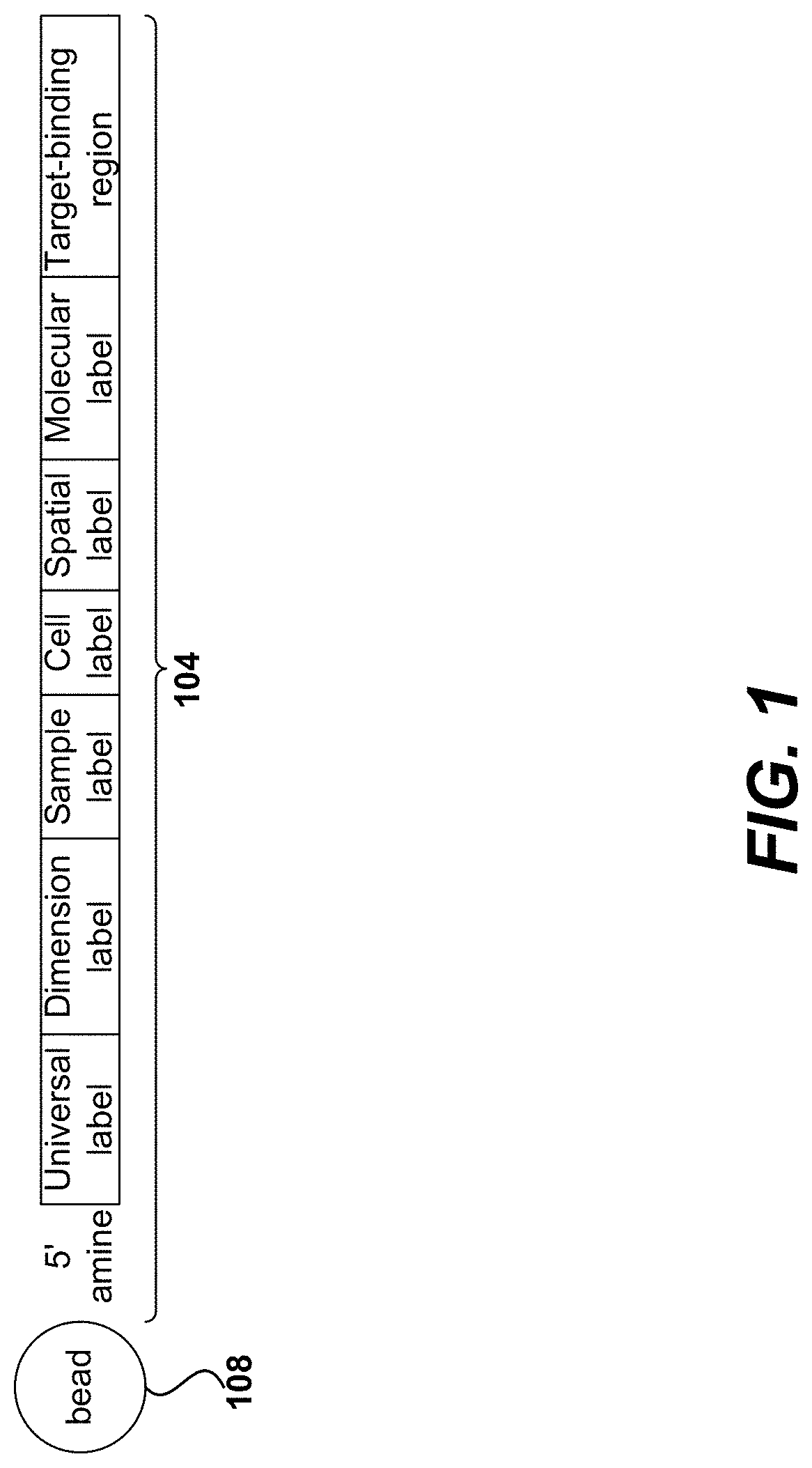
Systems, methods, compositions, and kits for measuring secreted factors from cells are disclosed herein, including those capable of determining single cell secretion activity and protein expression and / or gene expression simultaneously. Disclosed herein include bispecific probes comprising an anchor probe capable of specifically binding to a surface cellular target of a cell, and a capture probe capable of specifically binding to a secreted factor secreted by a cell that is associated with the capture probe. Also disclosed herein include secreted factor-binding reagents capable of specifically binding to a secreted factor bound by a capture probe, where a secreted factor-binding reagent can comprise a secreted factor-binding reagent specific oligonucleotide comprising a unique factor identifier sequence for the secreted factor-binding reagent.
Owner:BECTON DICKINSON & CO
Multiplexed single cell immunoassay
PendingUS20220178909A1Microbiological testing/measurementAssay labelsCell immunityCellular secretion
Disclosed herein include systems, methods, compositions, and kits for measuring the secretion level of a secreted factor of a single cell. Disclosed herein include solid supports comprising a plurality of capture probes capable of specifically binding to secreted factors secreted by a single cell. In some of the embodiments, at least two of the capture probes are capable of binding different secreted factors. Also disclosed herein include secreted factor-binding reagents capable of specifically binding to a secreted factor bound by a capture probe. Secreted factor-binding reagents can comprise a detectable moiety, or a precursor thereof. Secreted factor-binding reagents capable of binding the same secreted factor comprise the same detectable moiety, or a precursor thereof, and secreted factor-binding reagents capable of binding different secreted factors can comprise different detectable moieties, or precursors thereof.
Owner:BECTON DICKINSON & CO
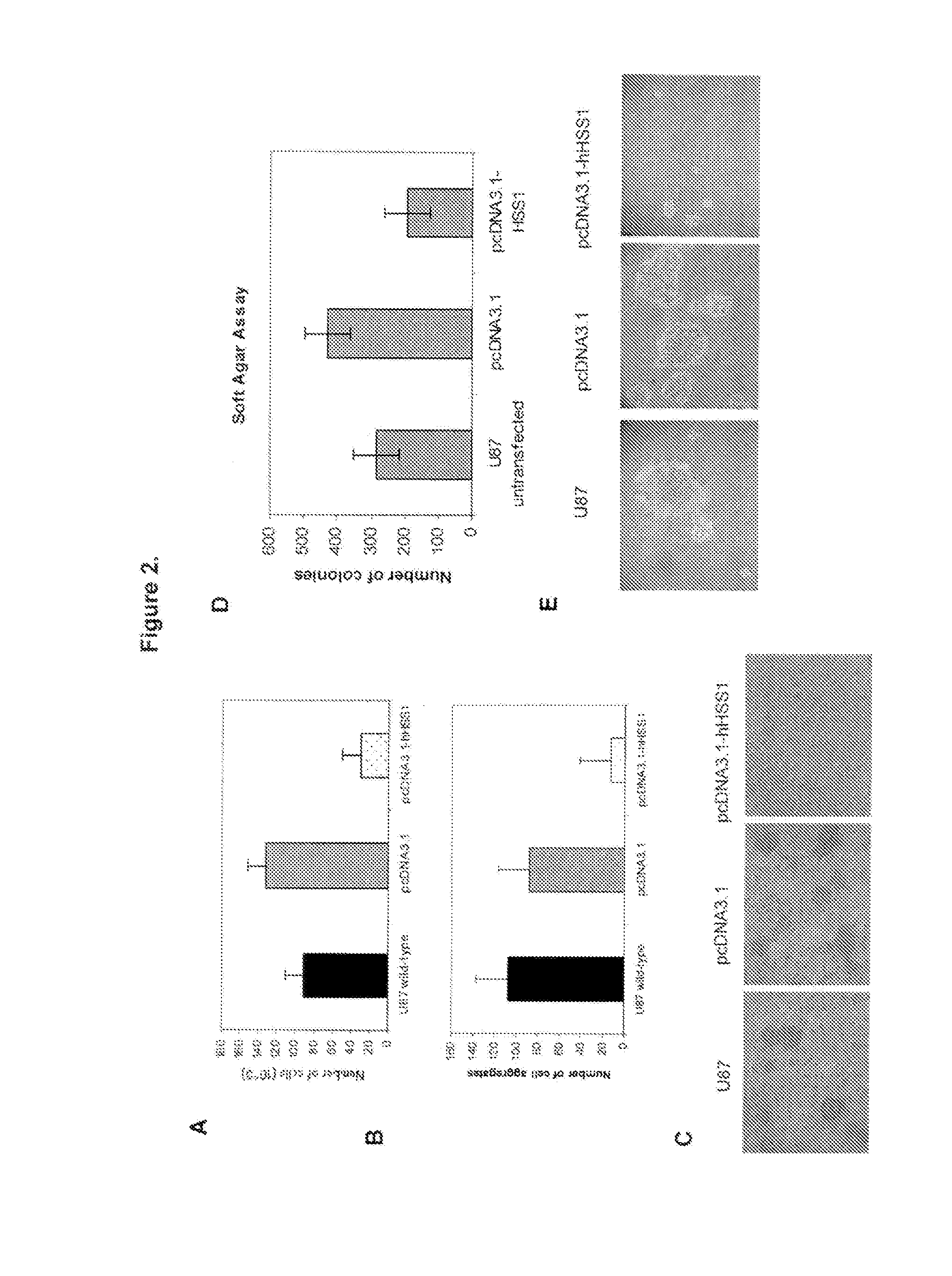
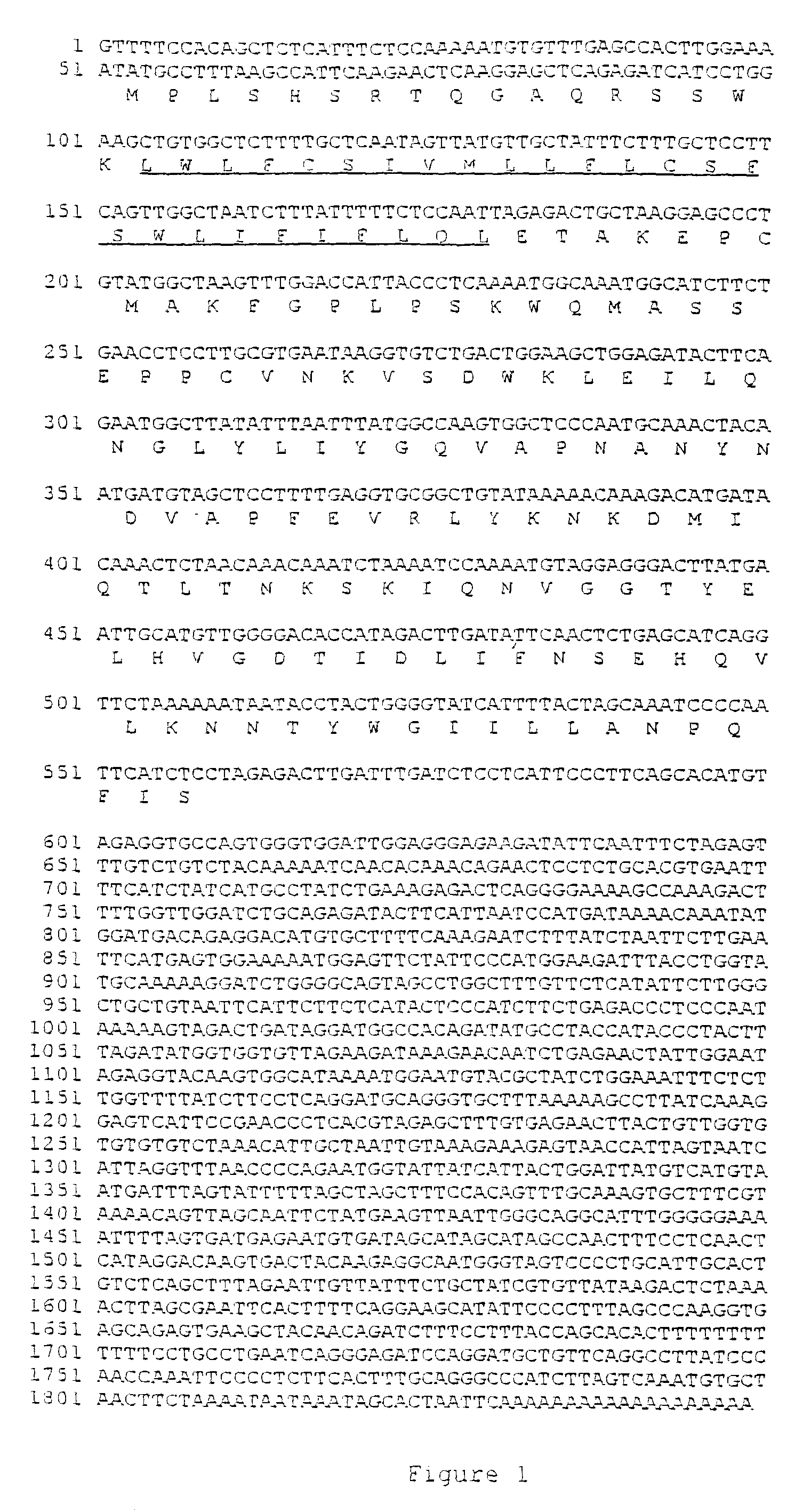
Method for treating brain cancer using a novel tumor suppressor gene and secreted factor
ActiveUS20130012452A1Improve survivalReduction in tumor massPeptide/protein ingredientsAntineoplastic agentsBrain cancersTumor suppressor gene
The present invention is directed to methods of using HSS1 (Hematopoietic Signal peptide-containing Secreted 1), HSM1 (Hematopoietic Signal peptide-containing Membrane domain-containing 1), or a combination thereof in the treatment of various cancers, such as brain cancers.
Owner:NEUMEDICINES INC
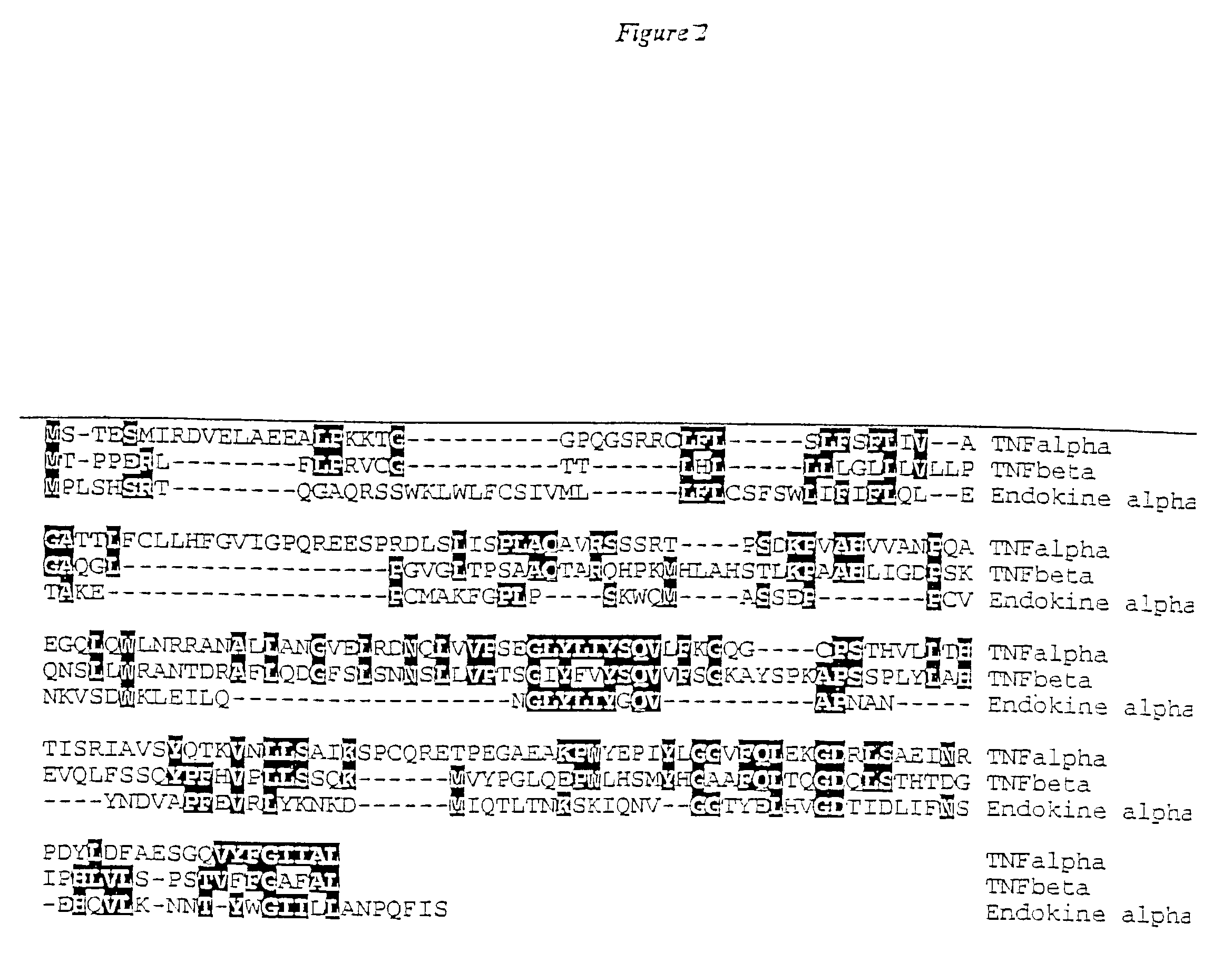
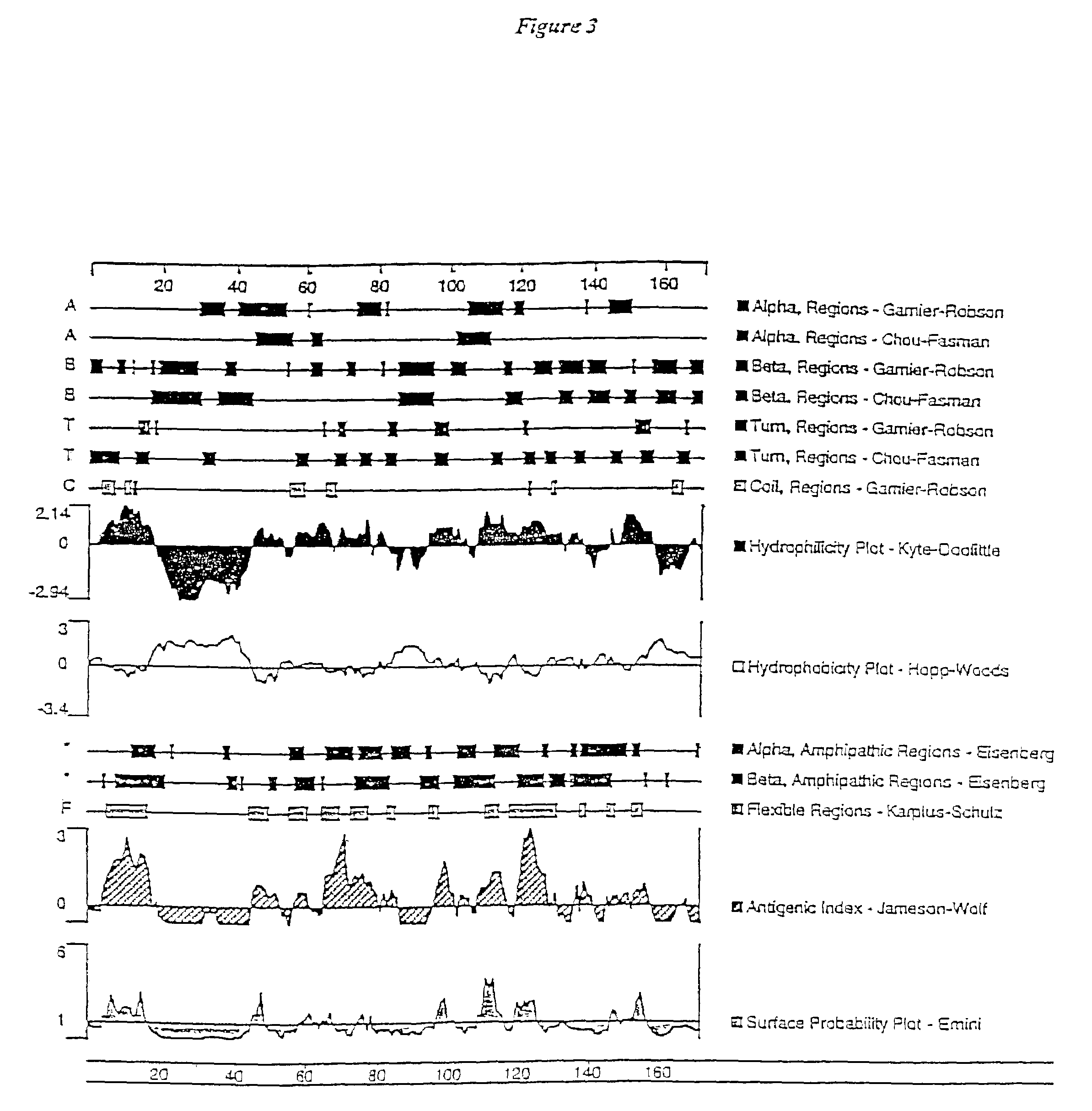
Methods and compositions for treating metabolic bone diseases relating to human endokine alpha
The present invention concerns methods for diagnosis and treatment of metabolic bone diseases and disorders using a novel member of the tumor necrosis factor (TNF) family of cytokines. In particular the invention provides methods of using the Endokine alpha protein and / or homomultimeric and / or heteromultimeric polypeptide complexes containing Endokine alpha, in the diagnosis, prognosis and treatment of metabolic bone diseases and disorders. Also provided by the invention are methods of using the Endokine alpha protein and / or homomultimeric and / or heteromultimeric polypeptide complexes containing Endokine alpha, in the diagnosis, prognosis and treatment of diseases and / or disorders associated with aberrant osteoclast development and / or activity. The present invention also provides isolated polynucleotides encoding polypeptides of the invention, antibodies thereto, and agonists and antagonists thereof, for use in the diagnosis, prognosis and treatment of metabolic bone diseases and disorders.
Owner:HUMAN GENOME SCI INC
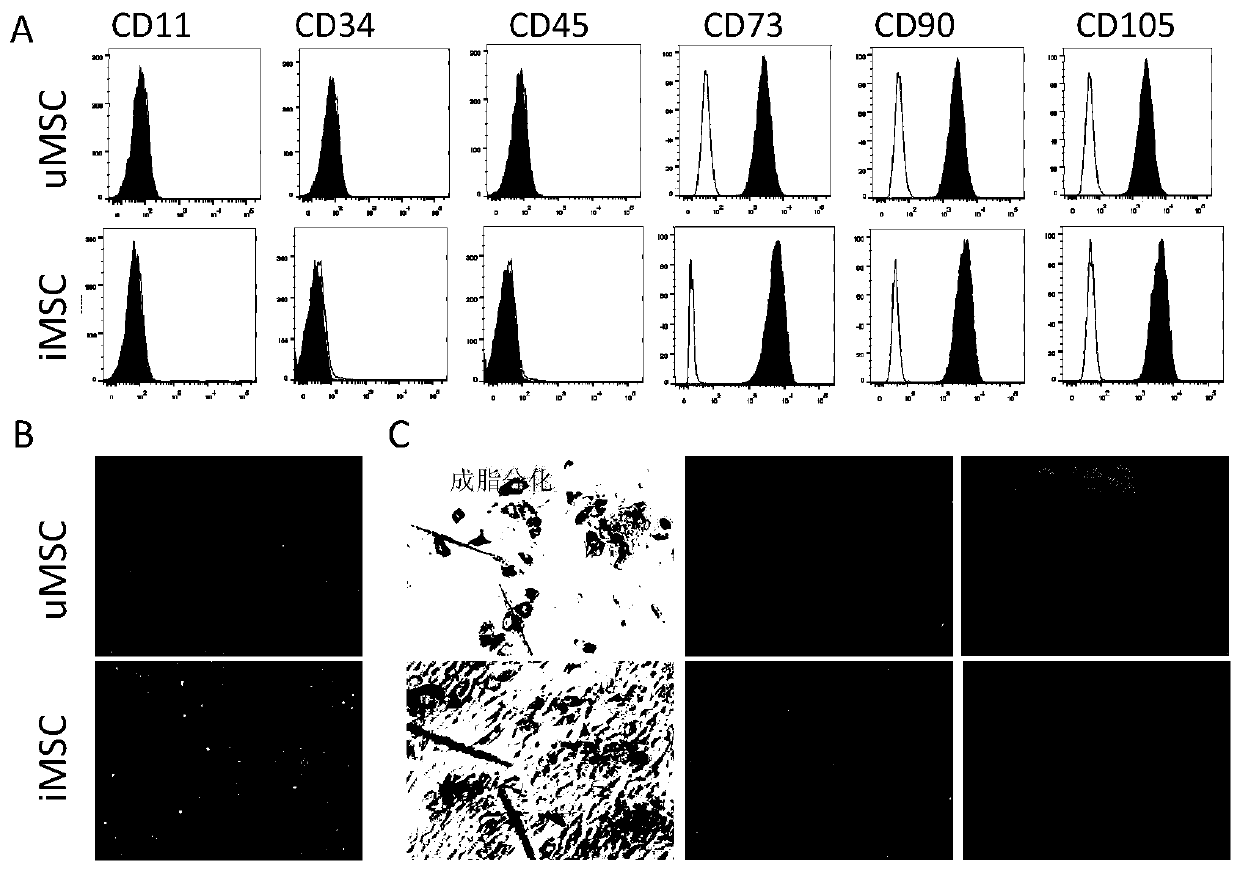
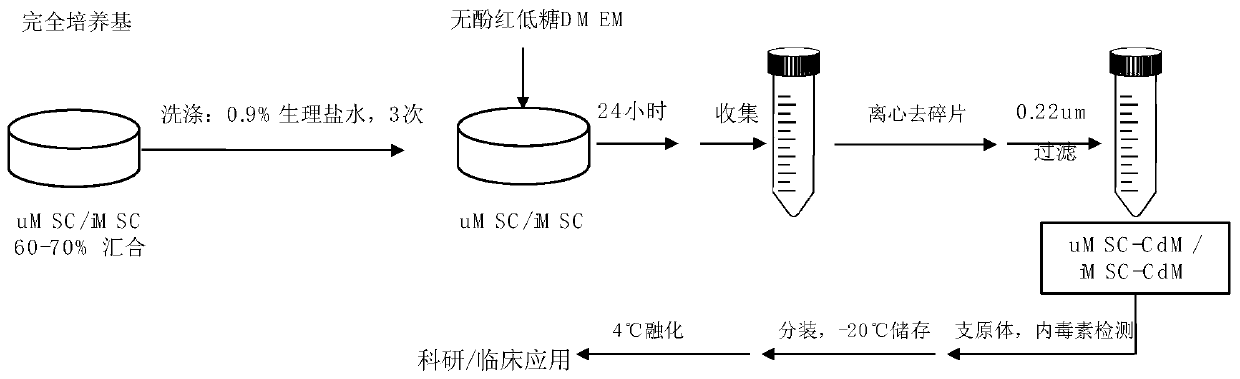
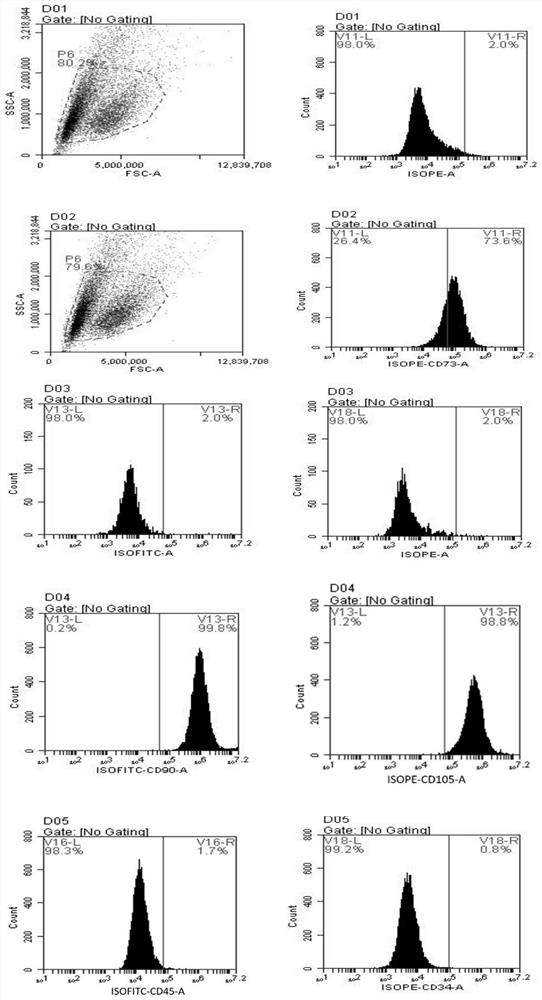
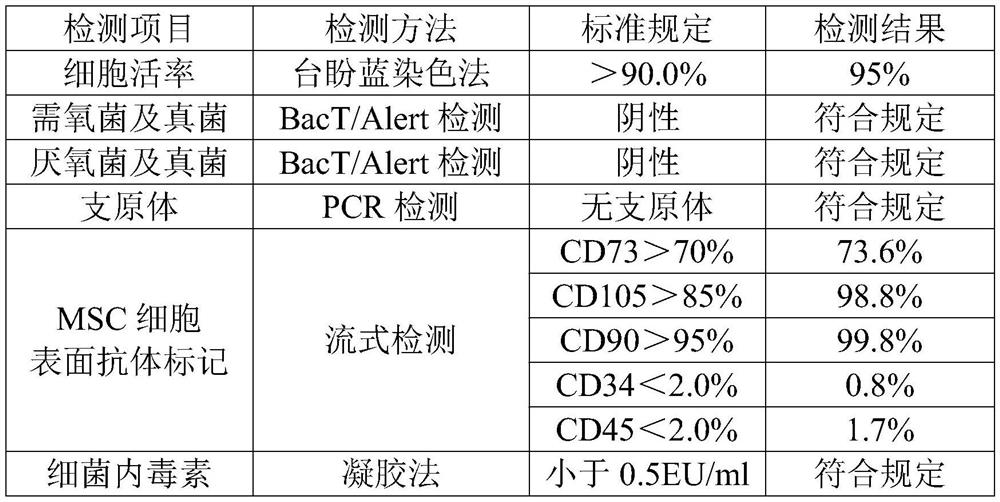
Preparation and application of mesenchymal stem cell secretion factors
ActiveCN110898078ASimple preparation processEasy to storeCell dissociation methodsCosmetic preparationsSkin repairBlood vessel
The invention provides a preparation method of mesenchymal stem cell secretion factors. Compared with mesenchymal stem cells from other sources, such as umbilical cord mesenchymal stem cells, the mesenchymal stem cells from pluripotent induced stem cells used in the invention are more stable in source and not easy to age after in vitro passage. The preparation method is simple in preparation process, the component of the obtained mesenchymal stem cells secrete factors is a human-derived stem cell secretion factor; the potential tumorigenic hidden danger of the stem cells is avoided; no exogenous additive or preservative is contained; no mycoplasma or endotoxin is contained; irritability is not easily caused; storage is convenient; better angiogenesis promoting capacity and skin defect healing promoting capacity are achieved; obvious promoting effect on vascular cell proliferation and angiogenesis is achieved; skin repair can be promoted, and skin aging can be delayed through external application; and a promising application prospect in skin injury treatment and development of beauty and skin care products is achieved. .
Owner:SHANGHAI EAST HOSPITAL EAST HOSPITAL TONGJI UNIV SCHOOL OF MEDICINE
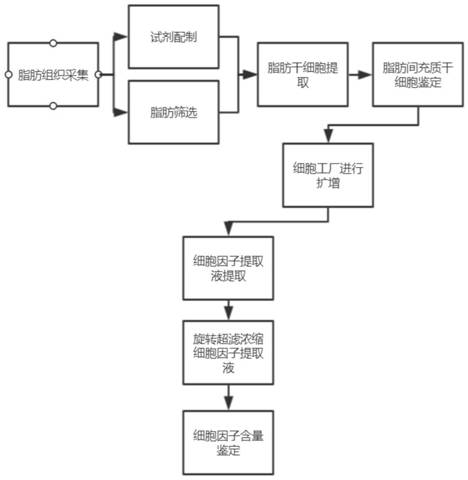
Method for extracting paracrine factor from adipose-derived stem cells
PendingCN112080465AStable karyotypeStable extraction efficiencyPeptide preparation methodsSkeletal/connective tissue cellsCD29Digestion
The invention discloses a method for extracting a paracrine factor from adipose-derived stem cells. The method comprises the following steps: taking a collected fat sample, and washing the fat samplewith normal saline for 1-3 times; adding a proper amount of adipose cell digestive juice for digestion; performing centrifugation, resuspending cell precipitate, and performing primary culture with aserum-free medium; performing subculture; performing enlarged culture; and performing extraction with a paracrine factor extracting solution, and performing purification to obtain the paracrine factor, wherein the paracrine factor extracting solution is prepared from a PBS buffer solution, L-glutamine, D-glucose and L-ascorbic acid, and the concentrations of the L-glutamine, the D-glucose and theL-ascorbic acid are 1-3mmol / L, 10-20 micromoles / L and 10-100 micromoles / L respectively. The method disclosed by the invention can stably extract the adipose-derived stem cells from adipose tissues, and extract the paracrine factor of the adipose-derived stem cells by utilizing the adipose-derived stem cells, so that the cell quality and the factor extraction efficiency are stable; and the adipose-derived stem cells are separated and cultured for a long time in a GMP environment, do not contact any animal-derived component, and can efficiently express adipose-derived stem cell markers such as CD29, CD44 and CD105.
Owner:北京银丰鼎诚生物工程技术有限公司
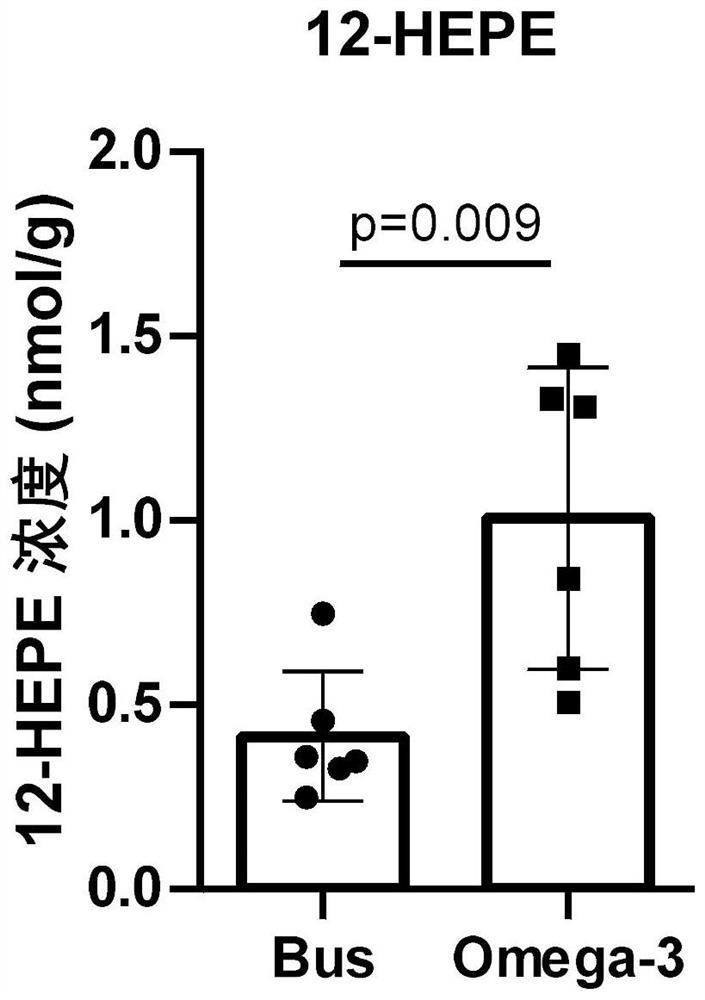
Application of 12-HEPE or pharmaceutically acceptable fatty acid thereof in alleviation of spermatogenesis disorder
ActiveCN113855659APromote proliferationGood treatment effectOrganic active ingredientsSexual disorderMouse TesticlePharmaceutical medicine
The invention discloses novel application of 12-HEPE or pharmaceutically acceptable fatty acid thereof, and comprises the application of the 12-HEPE or the pharmaceutically acceptable fatty acid thereof in preparation of a medicine for treating NOA. The 12-HEPE or the pharmaceutically acceptable fatty acid thereof can play a therapeutic role by promoting proliferation and differentiation of the endogenous spermatogonium of a mouse damaged by busulfan, and experiments prove that recovery of the thickness and the integrity of the spermatogenic epithelium of the testis and the recovery of the sperm concentration of the tail of epididymiscan be observed through intragastric administration of the 12-HEPE into the NOA mouse induced by the busulfan, meanwhile, proliferation and differentiation of spermatogonium are promoted, expression of the paracrine factors of cells is supported, so that the 12-HEPE can be used as a new target for alleviating the spermatogenesis dysfunction of the testis of the NOA mouse, and a new method is provided for treating the non-obstructive azoospermia.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A +3
Purification preparation method and application of mesenchymal stem cell secreted factors
ActiveCN113425619AEliminate wrinklesShrink poresCosmetic preparationsToilet preparationsSECRETOR FACTORChemistry
The invention provides a purification preparation method and an application of mesenchymal stem cell secreted factors. The purification preparation method comprises the following steps: (1) preparing MSC supernate; (2), purifying the mesenchymal stem cell secreted factors, including filtration and concentration, heparin affinity chromatography, and heparin affinity chromatography elution peak 5kD ultrafiltration or G-25 desalination column desalination; and (3) using the purified MSC secretion factors in the step (3) to prepare masks. According to the invention, a heparin affinity chromatography medium is adopted to specifically adsorb mesenchymal stem cell (MSC) secreted factors and purify substances with an effect of stimulating NIH 3T3 cell proliferation in MSC secretions, and the substances are potentially used for repairing refractory wounds and are added to cosmetics as an additive. The effects of repairing damaged skin, eliminating skin wrinkles, shrinking pores, improving complexion and the like can be achieved.
Owner:铜仁市泛特尔生物技术有限公司
Externally-applied medicine for treating cutaneous appendages and skin pruritic disease and preparation method and application thereof
InactiveCN106265737AFast curative effectImprove immune systemMammal material medical ingredientsDermatological disorderDamages skinDisease
The invention discloses an externally-applied medicine for treating cutaneous appendages and skin pruritic disease and a preparation method and application thereof. The main component of the medicine is an NK cell stock solution which is obtained by amplifying and culturing NK cells. The NK cell stock solution is mixed with different medicine auxiliaries, and an externally-applied liquid preparation or externally-applied paste preparation can be prepared. Compared with the prior art, the externally-applied medicine can be directly used for treating acne, seborrheic dermatitis, pruritus, prurigo nodularis and other cutaneous appendages and skin pruritic disease and is rapid in treatment effect and meanwhile can improve the skin immune system and repair damaged skin according to the characteristics that protein and cell factors secreted by the NK cells can be easily absorbed by skin and can directly act on target cells. Secreted factors in the NK cell stock solution can kill fungi and bacteria, and the medicine achieves the aim of comprehensively treating acne, dermatitis and pruritus, is wide treatment range and can prevent repeated aggravation of pathogenetic conditions.
Owner:SHANGHAI MINGDA BIOTECH CO LTD
Skin care product for antiallergic repair and preparation method thereof
PendingCN110974773AKeep alivePromote growthCosmetic preparationsToilet preparationsBiotechnologyFreeze-drying
Belonging to the technical field of cosmetics, the invention discloses a skin care product for antiallergic repair and a preparation method thereof. The skin care product for antiallergic repair comprises the following components: stem cell exocrine factor lyophilized powder, stem cell inclusion lytic factor lyophilized powder, hyaluronic acid, butanediol, an olive leaf extract, a purslane extract, an evening primrose extract, chamomile hydrolat and purified water. The stem cell secretion factor and stem cell inclusion lytic factor contained in the skin care product provided by the invention are prepared by low temperature freeze drying of a cell culture solution and a cell suspension, and the contained various stem cell secretion factors can promote the repair and regeneration of cells; the olive leaf extract, the purslane extract, the evening primrose extract and the chamomile hydrolat can relieve sensitive skin symptoms and improve skin resistance. The skin care product disclosed bythe invention can rapidly and effectively relieve anaphylactic reaction, improve the resistance of the body itself, and also can play a repair role.
Owner:谭啸
Culture method and cell cluster
Owner:PUBLIC UNIV CORP YOKOHAMA CITY UNIV +1
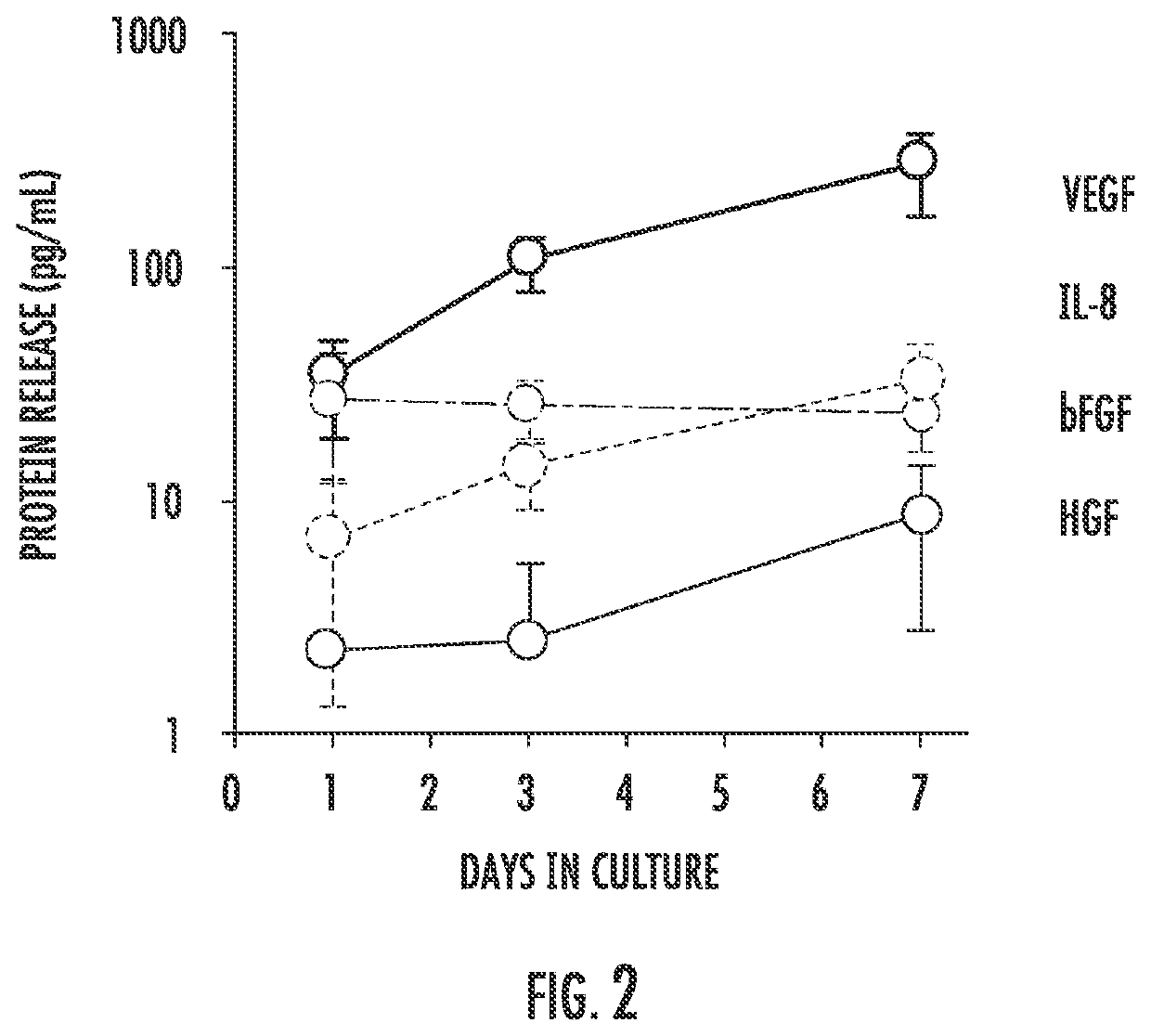
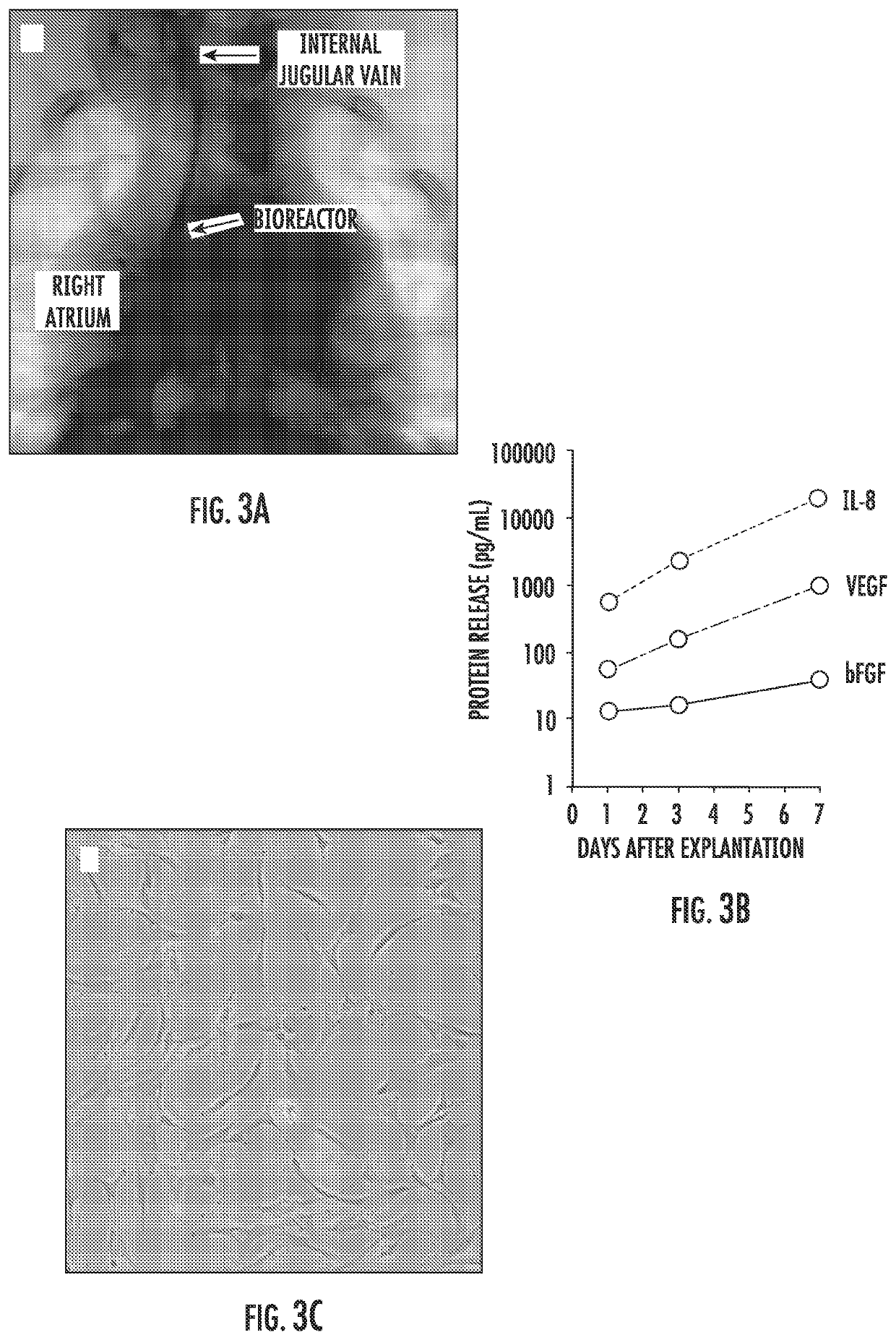
Implantable bioreactor and methods for making and using same
PendingUS20200297474A1Improve retentionConducive to survivalPeptide/protein ingredientsAerosol deliveryActive agentCell culture media
The present invention provides an implantable bioreactor comprising cells enclosed within an enclosure, said cells being capable of producing paracrine factors, wherein the enclosure is collapsible or expandable or both or neither, wherein the enclosure is semipermeable such that it provides containment of the cells preventing the egress of the cells while further providing a barrier that shields the cells from immunological attack, and wherein the enclosure is permeable to the entire secretome of the cell including exosomes, nucleic acids and proteins. The implantable bioreactor can have various configurations and can house internally a cell culture matrix than can include hydrogels, microbeads, and nanofiber matrices along with other active agents.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Preparation method of mesenchymal stem cell paracrine factor and application of paracrine factor
PendingCN113481152AIncrease the number ofIncrease concentrationCosmetic preparationsToilet preparationsCell culture mediaInterstitial cell
The invention provides a preparation method of a mesenchymal stem cell paracrine factor and application of the paracrine factor, and belongs to the technical field of cosmetics. The preparation method of the mesenchymal stem cell paracrine factor comprises the following steps that (1), mesenchymal stem cells are inoculated into an alpha-MEM culture medium containing 10% of fetal calf serum, the cell concentration is adjusted to be 1*10<7> L<-1>, culturing is carried out in a culture box, digesting and subculturing are carried out by using 0.2% pancreatin, the density of the obtained mesenchymal stem cells is greater than 90%, and the mesenchymal stem cells with a required number are obtained; (2), the mesenchymal stem cells are inoculated into an induction culture medium, a metformin aqueous solution is added into the induction culture medium, and culturing is carried out for 20-30 hours; (3), the serum-free interstitial cell culture medium in the step (2) is replaced, and starvation treatment is carried out; and (4), the cell culture fluid in the step (3) is collected and concentrated, and vacuum freeze-drying is carried out to obtain powder.
Owner:中科博赛干细胞再生医学科技广州有限公司
Mesenchymal stem cell substitute product containing degradable biomedical sustained-release material and application thereof
InactiveCN108815509ANo risk of infectionImprove functional statusOrganic active ingredientsPeptide/protein ingredientsMicrosphereFreeze-drying
The invention discloses a mesenchymal stem cell substitute product containing a degradable biomedical sustained-release material and application thereof. The mesenchymal stem cell substitute product comprises the following ingredients (by weight): 1-5% of the degradable biomedical sustained-release material, 0.1-5% of a mesenchymal stem cell secreted factor and the balance an electrolyte injection. The degradable biomedical sustained-release material is a medicinal raw material with plasticity, biocompatibility, histocompatibility and degradation product safety. The sustained-release materialis processed into slow-release gel or sustained-release microspheres wit different particle sizes. By a swelling blending method and / or a high voltage electrostatic method, the prepared ''mesenchymalstem cell paracrine mimetic'' containing the biomedical sustained-release material and the secreted factor has good biocompatibility and slow release performance, has no cytotoxicity, can promote corresponding cell proliferation, has good freeze-drying stability, can be directly stored at low temperature, is convenient to store and transport, helps reduce pollution risk caused by man-made operation, and can be directly used after being unfrozen.
Owner:TIANJIN AMCELLGENE ENG
Preparation method of paracrine factor inclusion with skin cell rejuvenation effect
PendingCN112773759AExtend your lifeStable in natureCosmetic preparationsToilet preparationsUmbilical cord tissueBlood vessel
The invention discloses a preparation method of a paracrine factor inclusion with a skin cell rejuvenation effect. The preparation method comprises the following steps of: step a, putting an umbilical cord into a storage and transportation bottle containing a storage and transportation liquid for later separation and preparation; b, placing the umbilical cord into a culture dish, fully washing the umbilical cord with normal saline, cutting off 1cm of the two ends of the umbilical cord, repeatedly washing the umbilical cord tissue until no obvious blood clots exist, cutting the umbilical cord into small sections with the length of 2cm, washing for three times, longitudinally splitting the umbilical cord, removing blood vessels, tearing off Wharton's jelly, fully washing the Wharton's jelly with normal saline for three times, and then cutting the tissue into tissue blocks with the size of 1-3 cubic millimeters. According to the method, the paracrine factors of the mesenchymal stem cells are wrapped with the microcapsules, can be stored at normal temperature and have stable properties, and the microcapsule inclusion and the fibroblasts are co-cultured, so that the increase of collagen secreted by the fibroblasts can be improved, the division activity can be improved, and the service life of the fibroblasts can be prolonged.
Owner:朱瑜
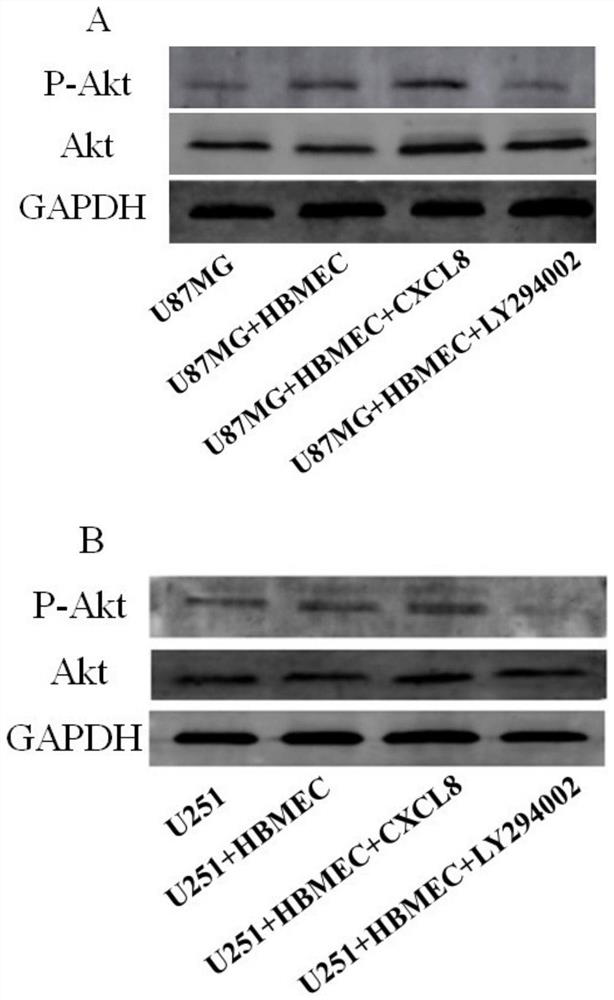
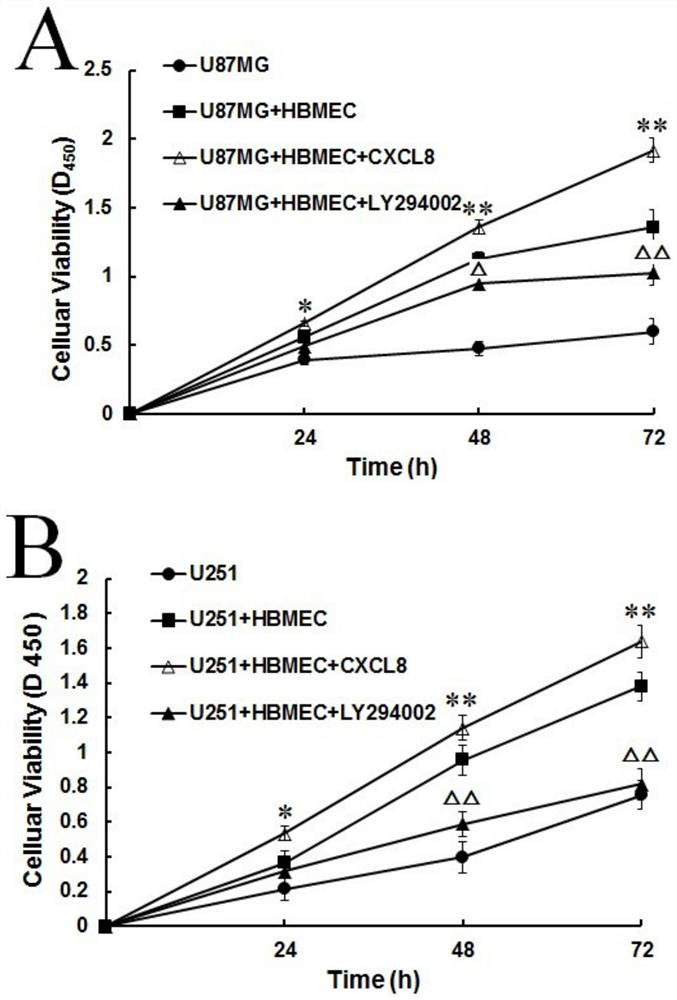
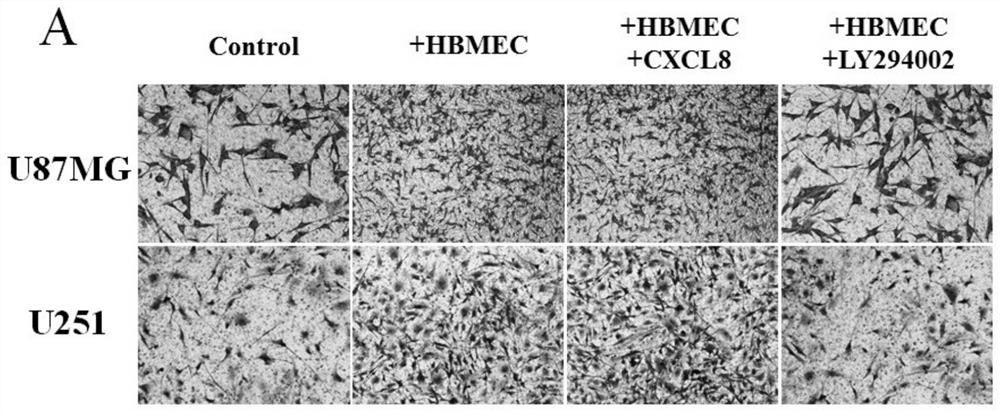
Research method of Crosstalk mechanism of VEGF-CXCL8-Akt and application thereof
PendingCN112379101AIncreased proliferationIncrease aggressivenessCompound screeningApoptosis detectionLY294002U87
The invention discloses a research method of a Crosstalk mechanism of VEGF-CXCL8-Akt and application thereof. The research method comprises the following steps of firstly, detecting the influence of U87MG, U251 and secretion factors VEGF thereof on secretion of CXCL8 by HBMEC by using ELISA, secondly, respectively adding a specific agonist CXCL8 and an inhibitor LY294002 of a CXCL8-Akt signal pathinto a co-culture system, and detecting the influence of co-culture and activation / inhibition on expression of Akt proteins of U87MG and U251 cells and phosphorylation activation state P-Akt thereofby using Western blotting, and then using CCK-8 and Transwell invasion experiments for respectively detecting the influence of co-culture and activation / inhibition on proliferation and invasion of U87MG and U251 cells.
Owner:NINGXIA MEDICAL UNIV +1
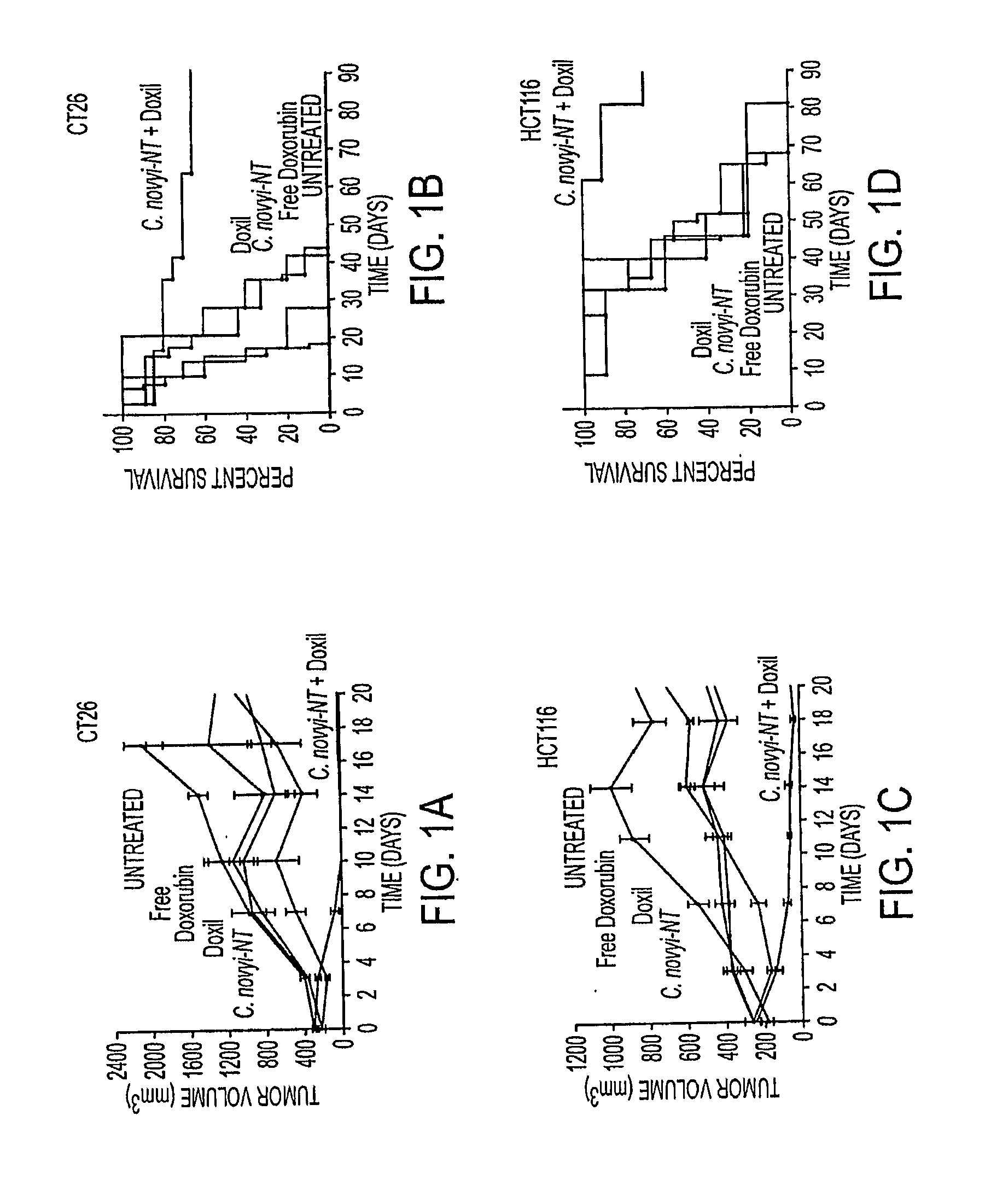
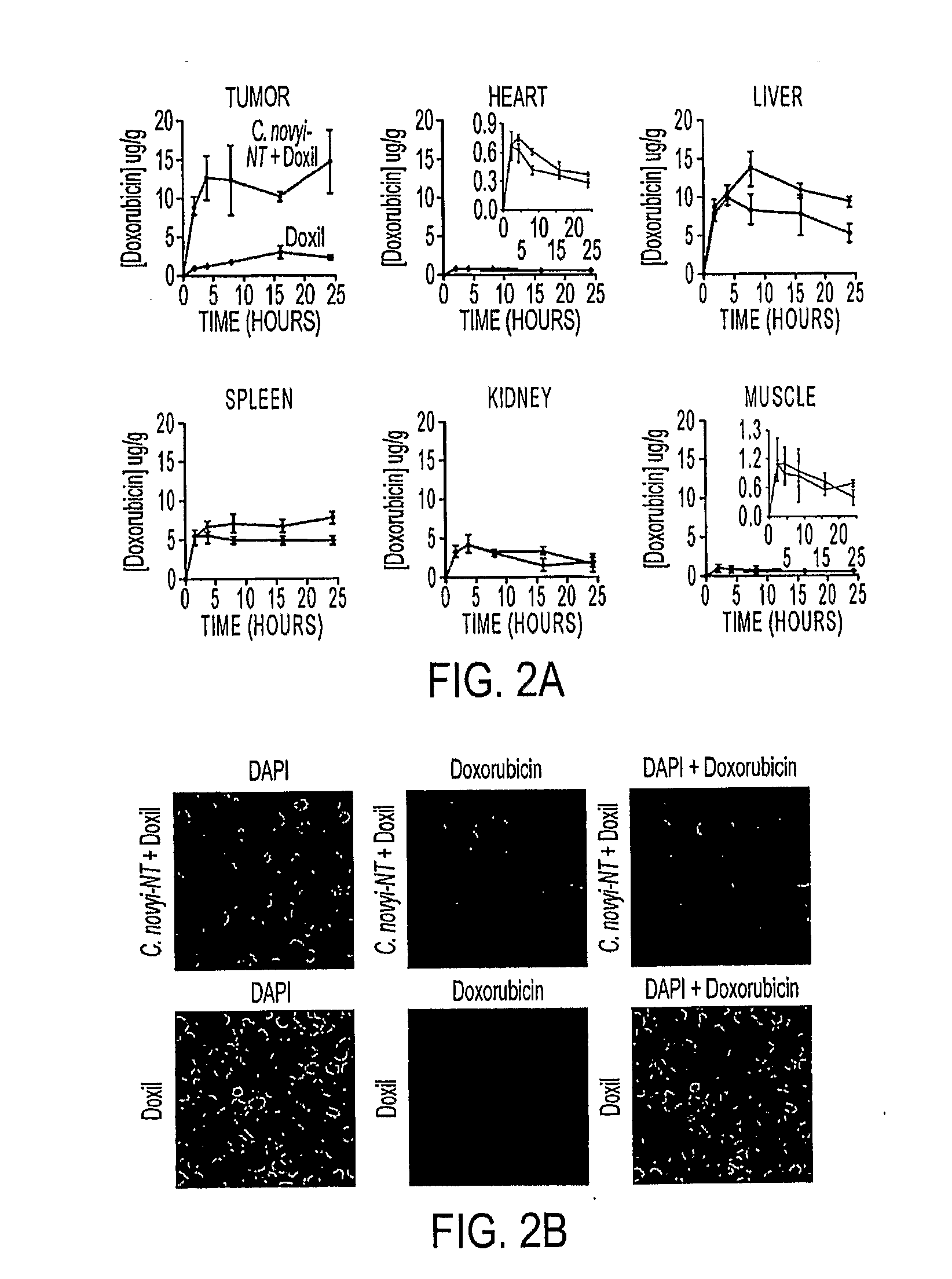
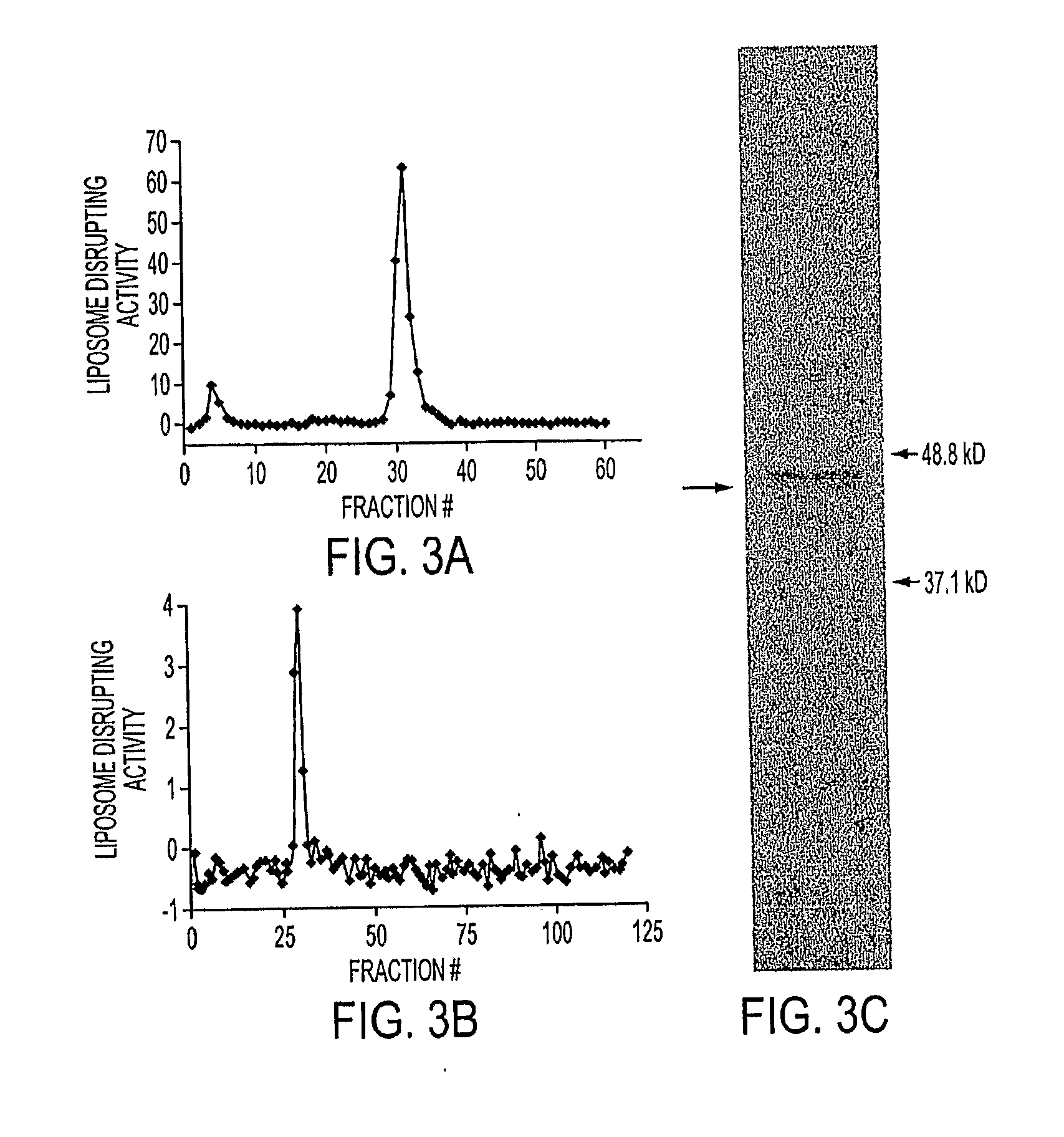
Tumor-specific delivery of therapeutic agents via liposomase
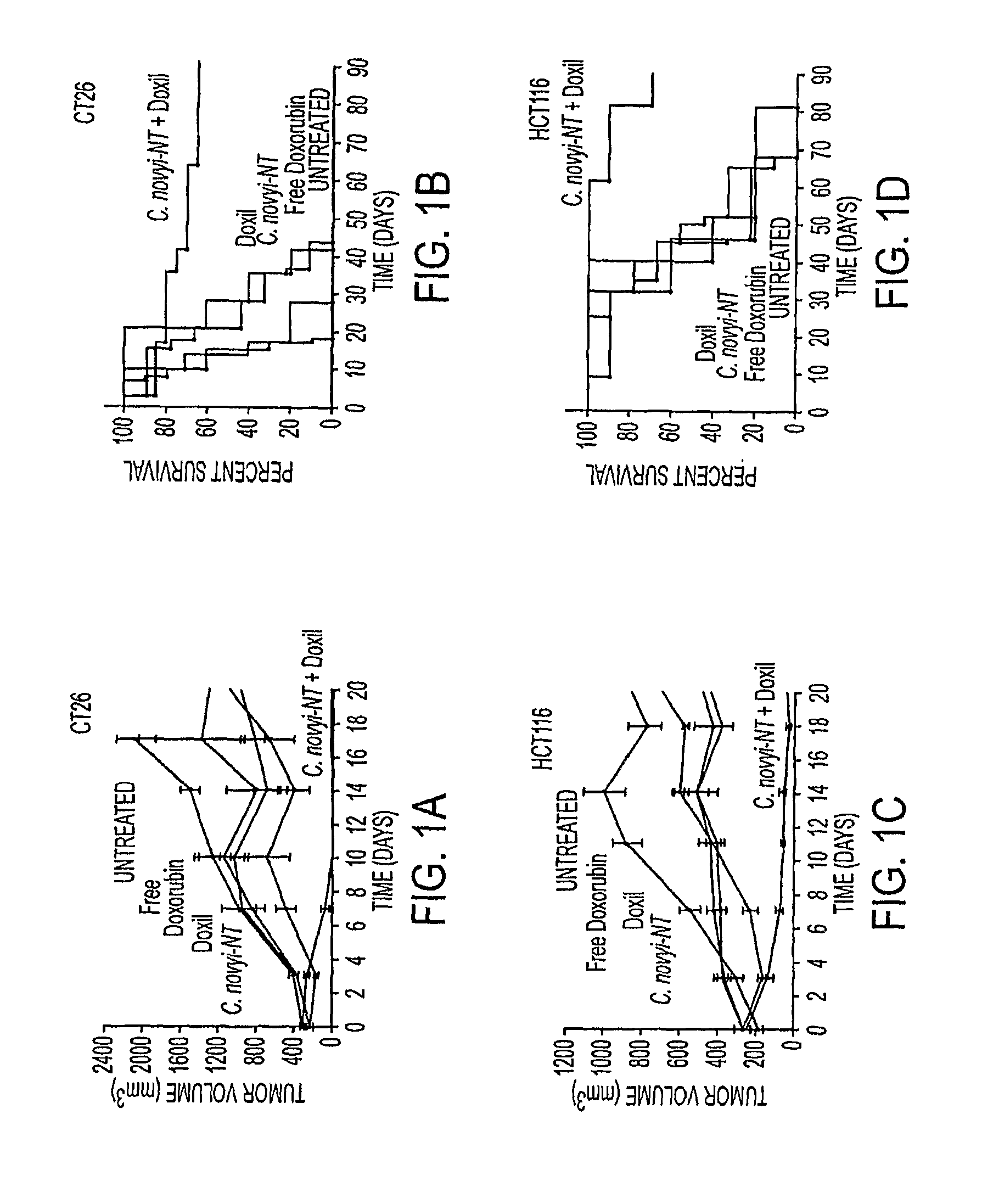
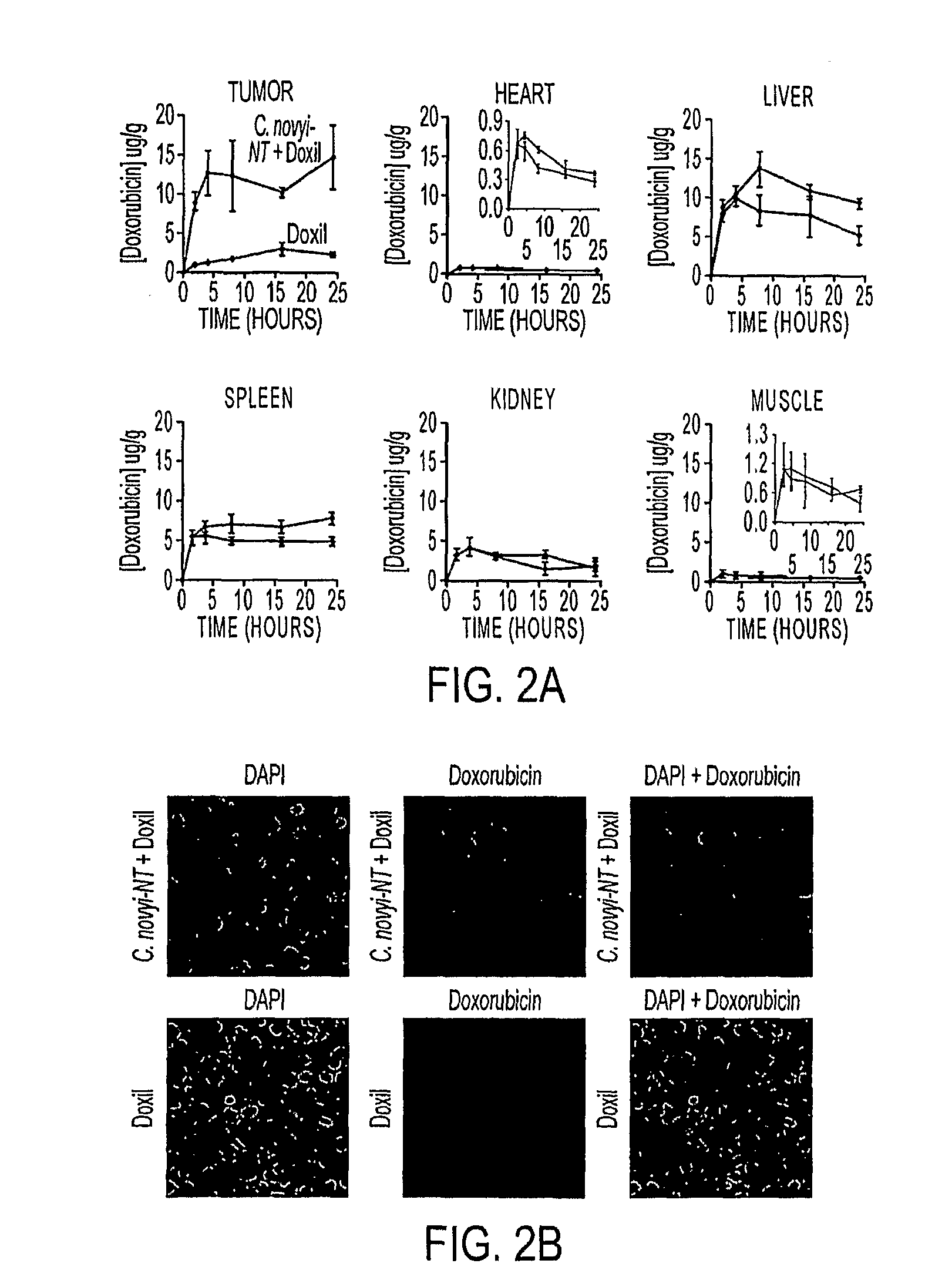
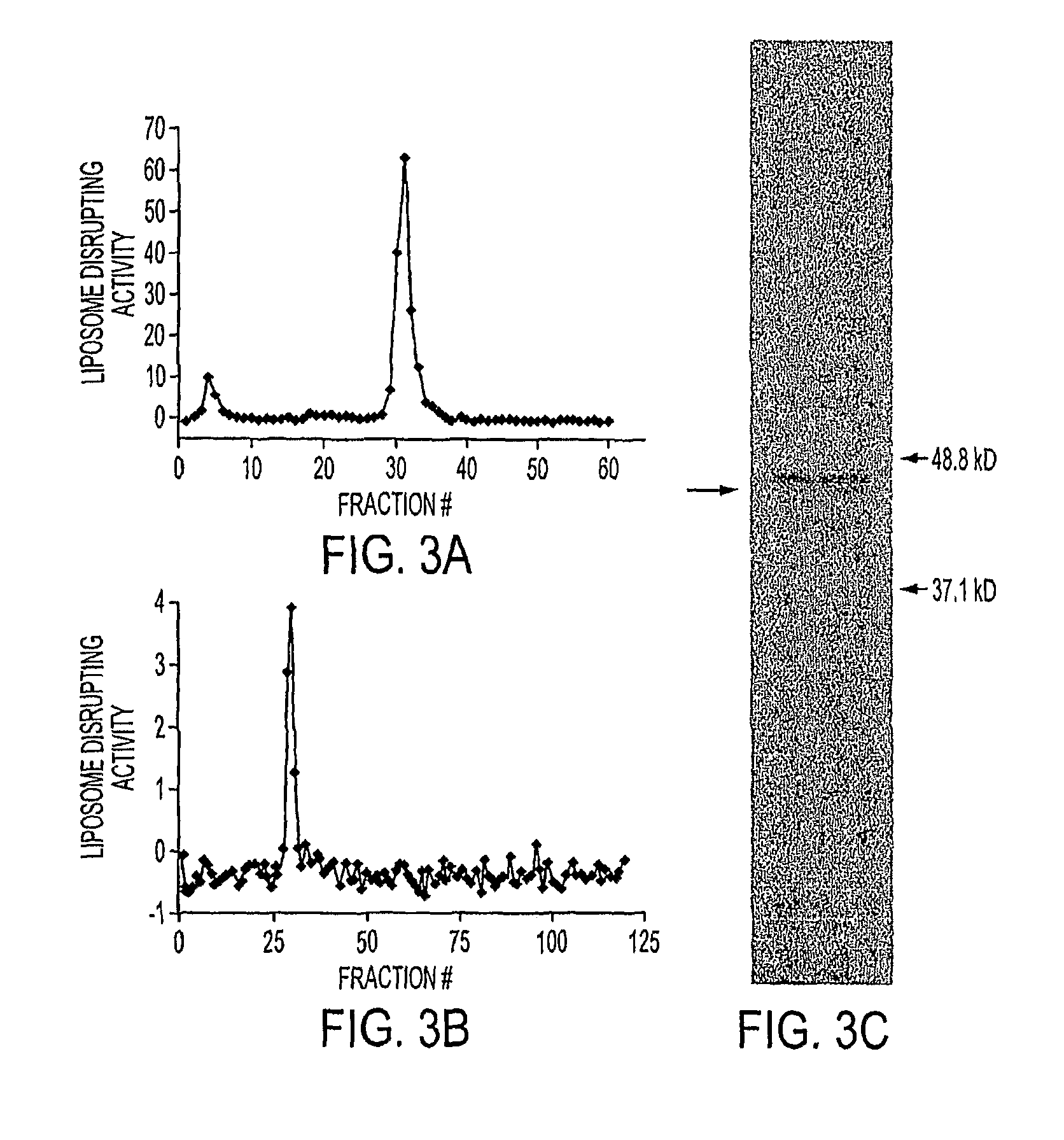
ActiveUS20110250258A1Wide applicabilityOrganic active ingredientsBiocideObligate anaerobeTherapeutic effect
Clostridium novyi is an obligate anaerobe that can infect hypoxic regions within experimental tumors. We found that mice bearing large, established tumors were often cured when treated with C. novyi plus a single dose of liposomal doxorubicin. The secreted factor responsible for this phenomenon was identified and, surprisingly, proved to be a member of the lipase family. The gene encoding this protein, called liposomase, has the potential to be incorporated into diverse therapeutic methods to deliver specifically a variety of chemotherapeutic agents to tumors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
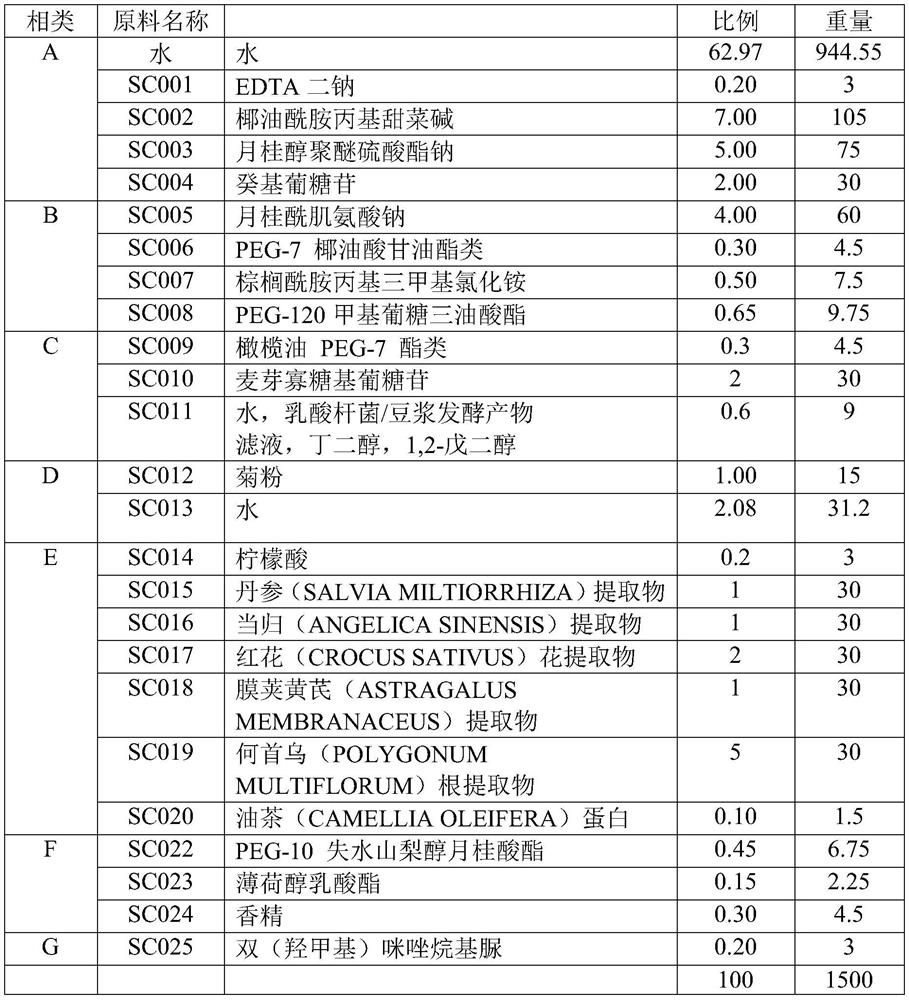
Anti-hair loss and hair growth promoting shampoo and preparation method thereof
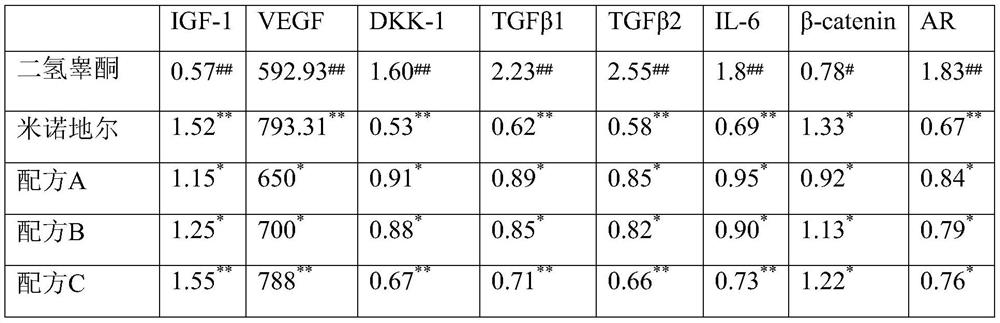
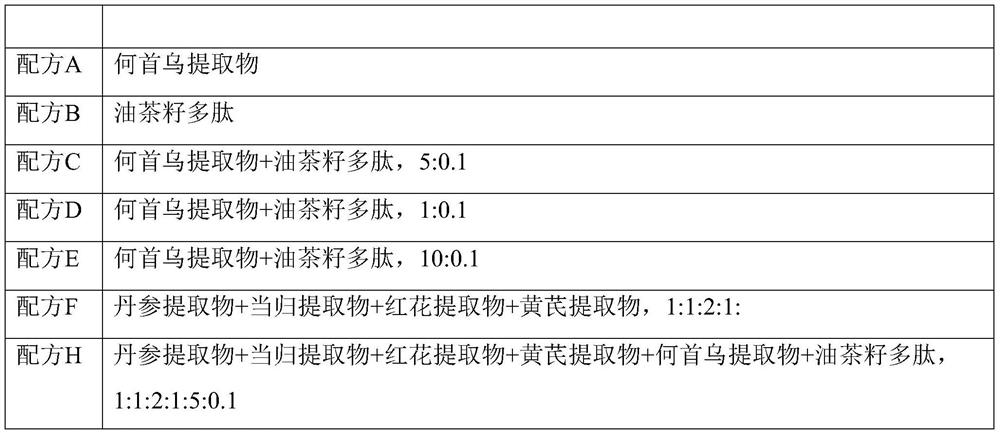
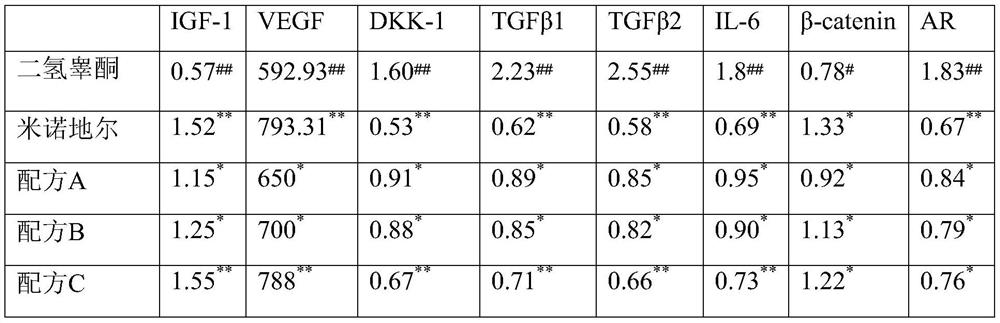
The invention discloses anti-hair loss and hair growth promoting shampoo and a preparation method thereof. The shampoo comprises active ingredients, wherein the active ingredients comprise a salvia miltiorrhiza extract, an angelica sinensis extract, a safflower extract, an astragalus membranaceus extract, a polygonum multiflorum extract and camellia seed polypeptide. The active ingredients consisting of the salvia miltiorrhiza extract, the angelica sinensis extract, the safflower extract, the astragalus membranaceus extract, the polygonum multiflorum extract and the camellia seed polypeptide can improve the activity of dermal papilla cells treated by dihydrotestosterone. In-vitro experiments show that secretion factors IGF-1, VEGF, DKK-1, TGF beta 1, TGF beta 2, IL-6 and the like of the dermal papilla cells as well as beta-catenin and AR return to normal levels. Patients suffering from hair loss and sparse hair can use the shampoo disclosed by the invention once every four days. The effective rate of patients suffering from hair loss is 85%, and the effective rate of patients with sparse hair is 98%.
Owner:山茶元素(北京)科技研发有限公司
Tumor specific delivery of therapeutic agents via liposomase
Clostridium novyi is an obligate anaerobe that can infect hypoxic regions within experimental tumors. We found that mice bearing large, established tumors were often cured when treated with C. novyi plus a single dose of liposomal doxorubicin. The secreted factor responsible for this phenomenon was identified and, surprisingly, proved to be a member of the lipase family. The gene encoding this protein, called liposomase, has the potential to be incorporated into diverse therapeutic methods to deliver specifically a variety of chemotherapeutic agents to tumors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Preparation method of adipose tissue-derived stem cell secreted factor
PendingCN114515296ARegulate vitalityGood synergistic effectCulture processUnknown materialsTissue repairTryptase
The invention discloses a preparation method of adipose-derived mesenchymal stem cell secreted factors, and relates to the technical field of mesenchymal stem cell secreted factor extraction, the preparation method comprises the following steps: 1, cell recovery; step 2, subculture: carrying out subculture on the adipose-derived stem cells by using a subculture medium; step 3, harvesting secretory factors; the subculture medium in the step 2 is prepared from 400 to 600 ml / L of stem cell basal culture medium, 1 to 3 mmol / L of L-alanyl-L-glutamine, 10 to 20 [mu] mol / L of 4-2-hydroxyethyl-1-piperazine ethanesulfonic acid, 2.0 to 6.0 [mu] g / mL of insulin, 10 to 20 g / L of D-glucose, 10 to 100 [mu] mol / L of L-ascorbic acid, 30 to 50 [mu] g / mL of all-transretinoic acid, 0.3 to 0.8 [mu] g / mL of serum protein, 0.1 to 0.3 [mu] g / mL of tryptase and 10.0 to 20.0 [mu] g / mL of ginsenoside. The adipose-derived mesenchymal stem cell secreted factor obtained in the application process has good stability, is not easily influenced by allergens, and can exert a good and stable tissue repair effect.
Owner:和携科技有限公司
Preparation method and application of targeting mesenchymal stem cell (MSC) drug delivery system
InactiveCN110777119AWide variety of sourcesAvoid scourGenetically modified cellsSkeletal/connective tissue cellsBiotechnologySMAD
The invention discloses a preparation method and application of a targeting mesenchymal stem cell (MSC) drug delivery system. The preparation method comprises the steps as follows: S1, stem cell collection; S2, cell detection; S3, production of a transfection mixed solution; S4, pretreatment of MSCs; and S6, alkaline culture. The MSCs are widely sourced, can be xenogenous as well as autogenous andhave sufficient sources, and the cells have excellent capacity in proliferation, survival, differentiation, migration, synthesis, factor secretion and other aspects, are free of ethical problems andhave low immunogenicity and broad application prospects. The disclosed tissue damage targeting MSCs can be effectively planted at tissue damage parts, blood washing or natural falling can be avoided,the homing cell quantity can be increased, besides, low expression of smad protein can be avoided, and the fibrosis promotion risk due to transplantation of the MSCs can be avoided.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
Application of mesenchymal stem cell paracrine factor to preparation of pain drugs
The invention discloses application of a mesenchymal stem cell paracrine factor to preparation of pain drugs, and relates to application of the technical field of biological stem cells to pain. One the one hand, the invention provides a preparation method of the mesenchymal stem cell paracrine factor; on the other hand, it is found that the paracrine factor prepared by the method can be applied tothe treatment of various kinds of pains, the specific pains comprise subjective sensation pains of all patients such as arthralgia, inflammatory pain and cancer pain, and the application prospect ofpreparing pain drugs is achieved. According to the mesenchymal stem cell paracrine factor, the pain caused by various reasons can be effectively alleviated; and a cell protein preparation has excellent biological effect on pain treatment.
Owner:北京中广天一生物科技有限公司
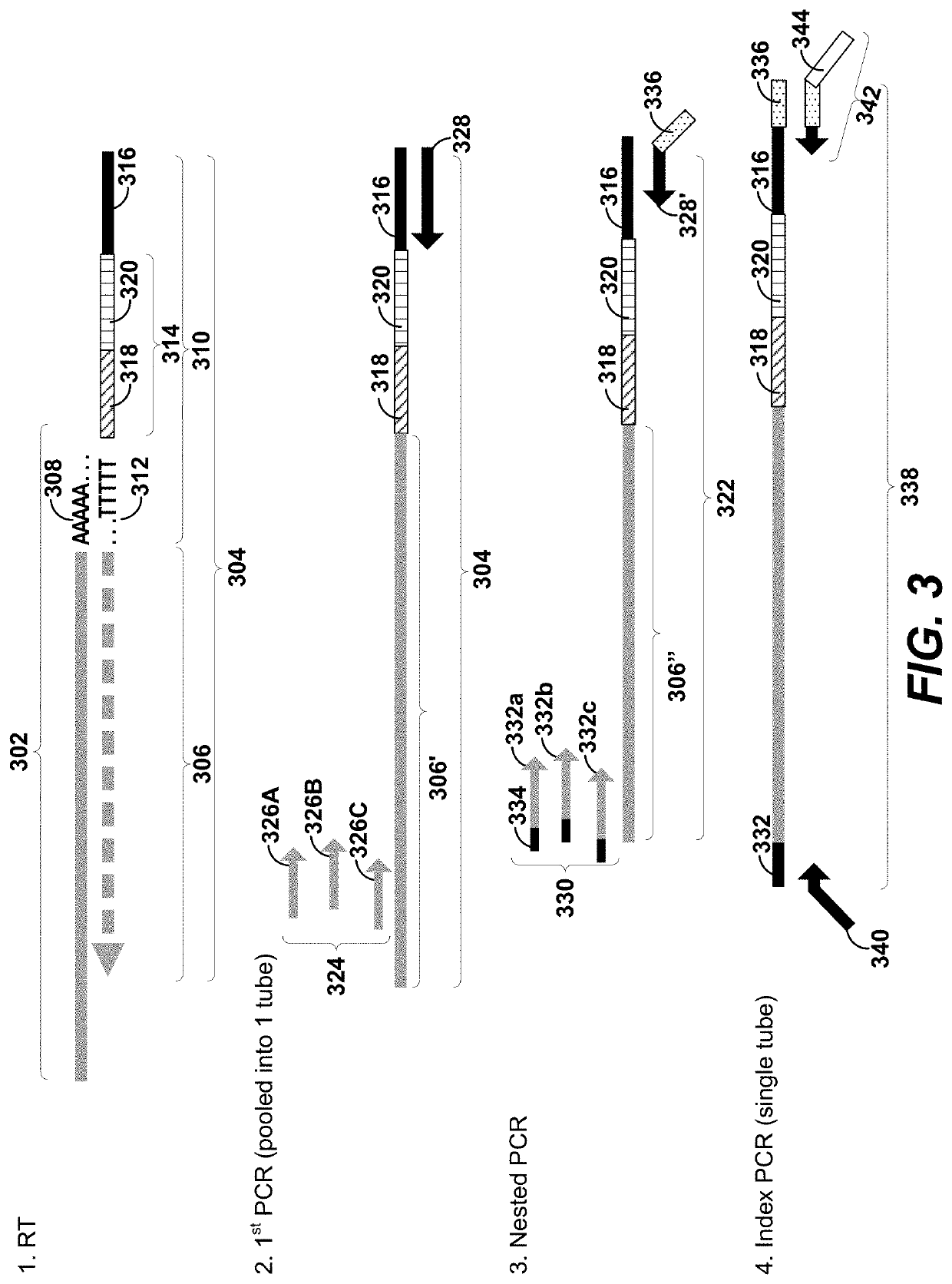
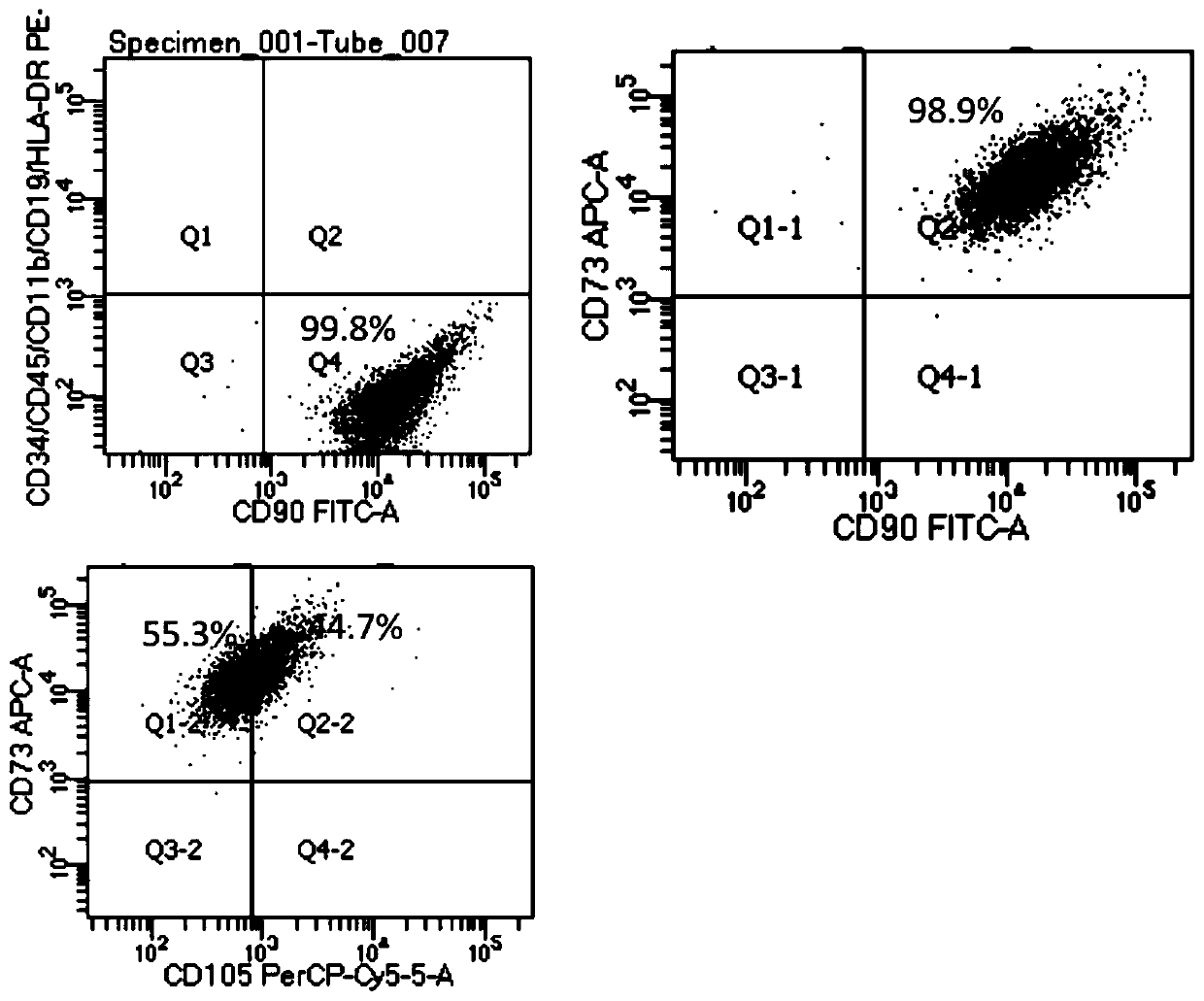
Single cell secretome analysis
PendingUS20220187286A1Microbiological testing/measurementBiological testingNucleotideCellular secretion
Systems, methods, compositions, and kits for measuring secreted factors from cells are disclosed herein, including those capable of determining single cell secretion activity and protein expression and / or gene expression simultaneously. Disclosed herein include solid supports comprising a plurality of capture probes capable of specifically binding to at least one of the plurality of secreted factors secreted by a single cell. Also disclosed herein include secreted factor-binding reagents capable of specifically binding to a secreted factor bound by a capture probe. A secreted factor-binding reagent can comprise a secreted factor-binding reagent specific oligonucleotide comprising a unique factor identifier sequence for the secreted factor-binding reagent.
Owner:BECTON DICKINSON & CO
Method for enhancing efficacy of stem cells by using ethionamide
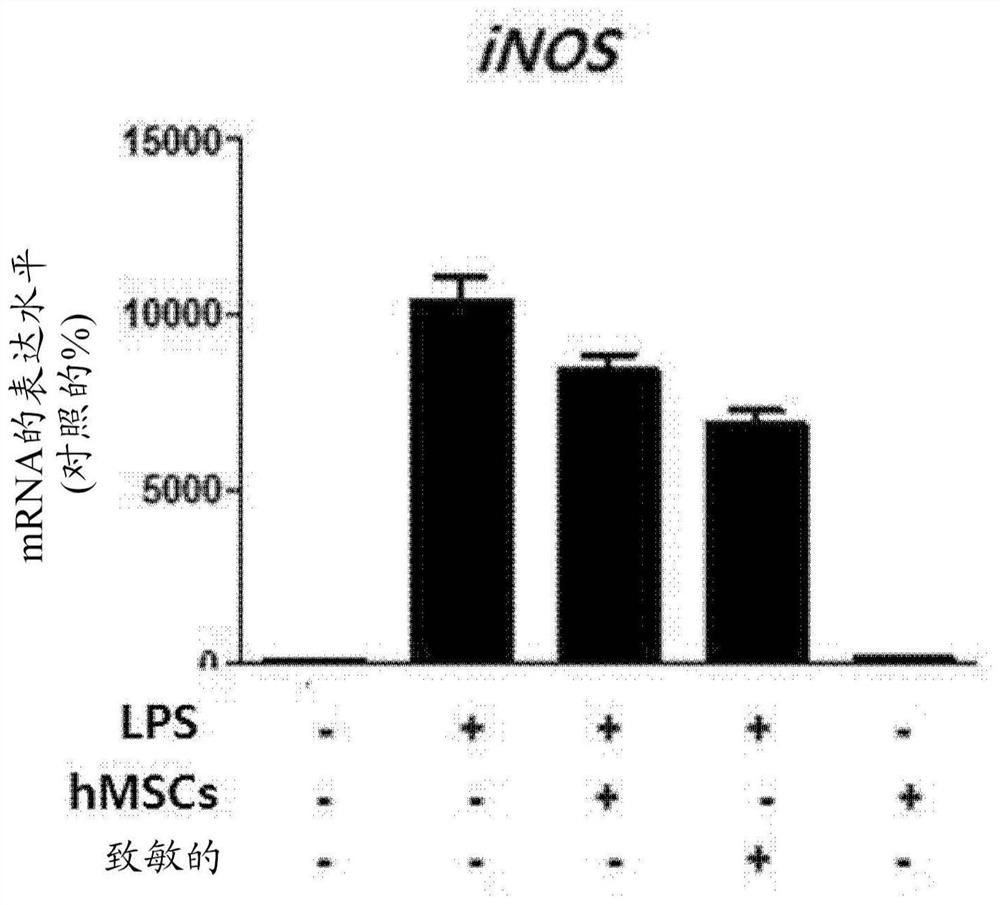
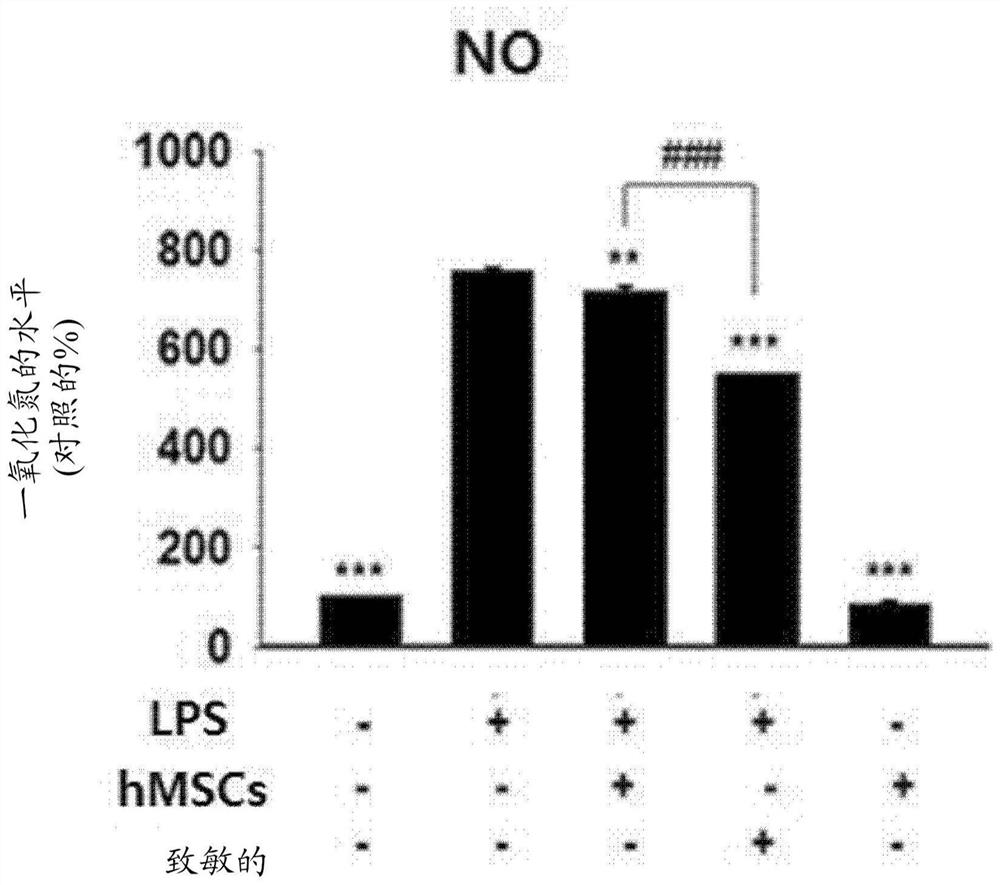
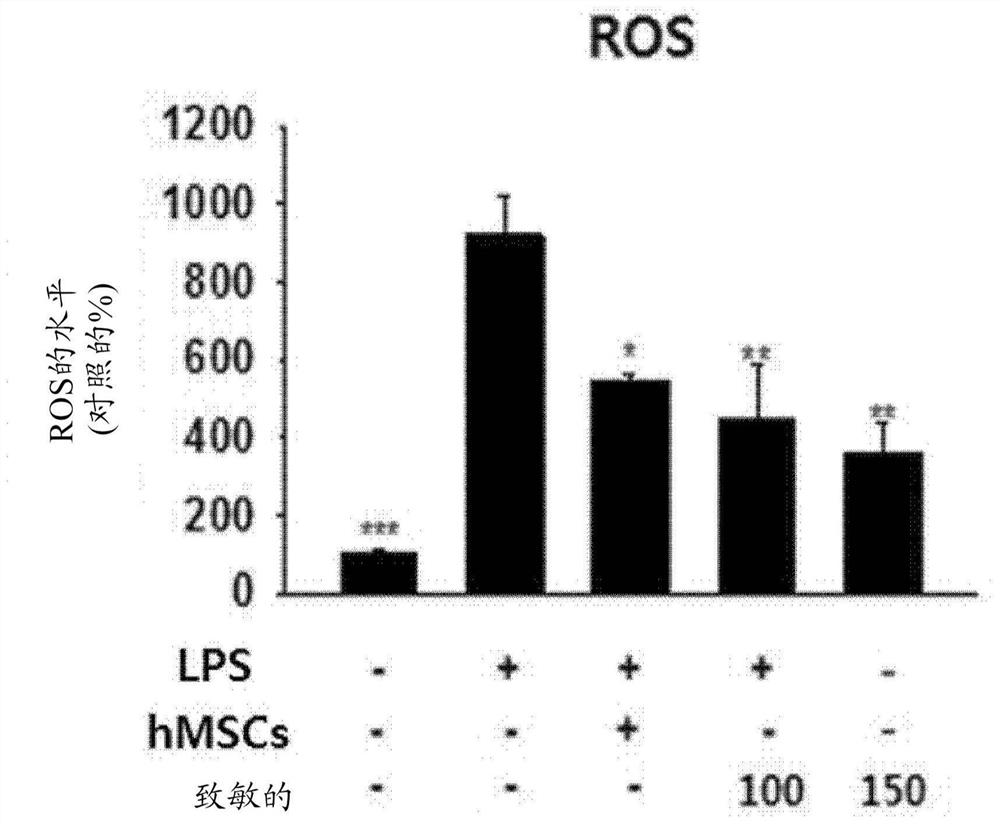
PendingCN114127265AIncreased paracrine activityImprove expression levelNervous disorderAntipyreticDiseaseMesenchymal stem cell
The present invention relates to a culture medium composition for enhancing the efficacy of stem cells, the culture medium composition comprising ethionamide; a method for enhancing the efficacy of stem cells and a method for producing stem cells having enhanced efficacy, comprising a step of culturing stem cells in the culture medium composition; stem cells produced by the method and uses thereof. According to the present invention, a simple process of treating mesenchymal stem cells with ethionamide can effectively enhance the anti-inflammatory action of the mesenchymal stem cells and the expression level of paracrine factors, and the stem cells obtained by the method can be used for preventing or treating inflammatory diseases or degenerative brain diseases.
Owner:SAMSUNG LIFE PUBLIC WELFARE FOUND
Stem cell composite preparation and preparation method thereof
PendingCN113230277AStrengthen resilienceChange textureOrganic active ingredientsCulture processNucleotideIdebenone
The invention discloses a stem cell composite preparation and a preparation method thereof, and belongs to the technical field of stem cell culture. The stem cell composite preparation comprises the following raw materials: a stem cell stock solution concentrated solution, non-crosslinked hyaluronic acid, water-soluble idebenone, nucleotide and vitamins. The yield of the stem cell paracrine related factors is improved through low-oxygen treatment, and non-crosslinked hyaluronic acid is introduced as a carrier, so that on one hand, the instant effect of the stem cell compound preparation in use is improved, and on the other hand, the diffusion speed of the stem cell paracrine factors to skin tissues is effectively controlled. Through adding the nucleic acid and idebenone, the synthesis of subcutaneous protein substances is increased, the antioxidant capacity of the skin is improved, the regulation of an endogenous skin cell microenvironment is changed into free radical resistance generated by exogenous ultraviolet rays, and the aim of rejuvenating the face is achieved on the whole.
Owner:陕西鸿瑞康生物科技有限公司
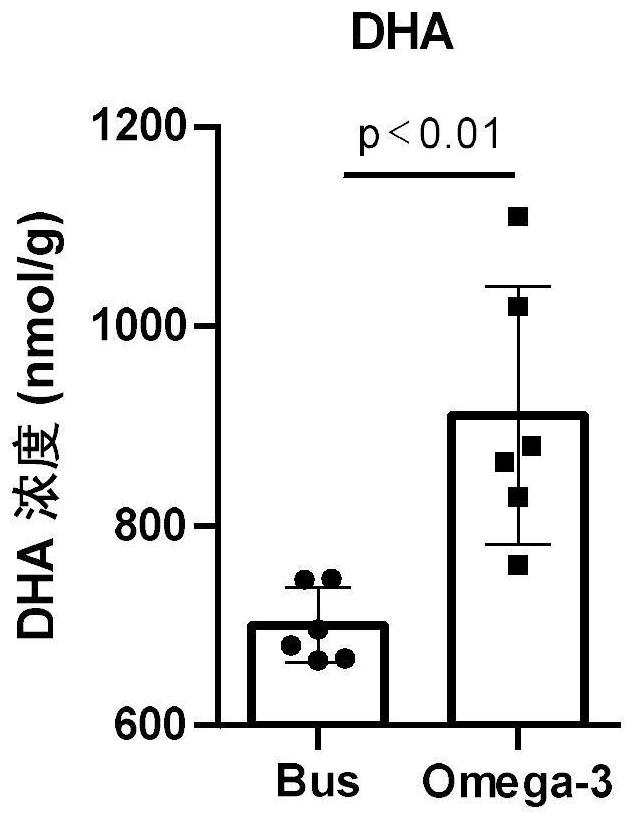
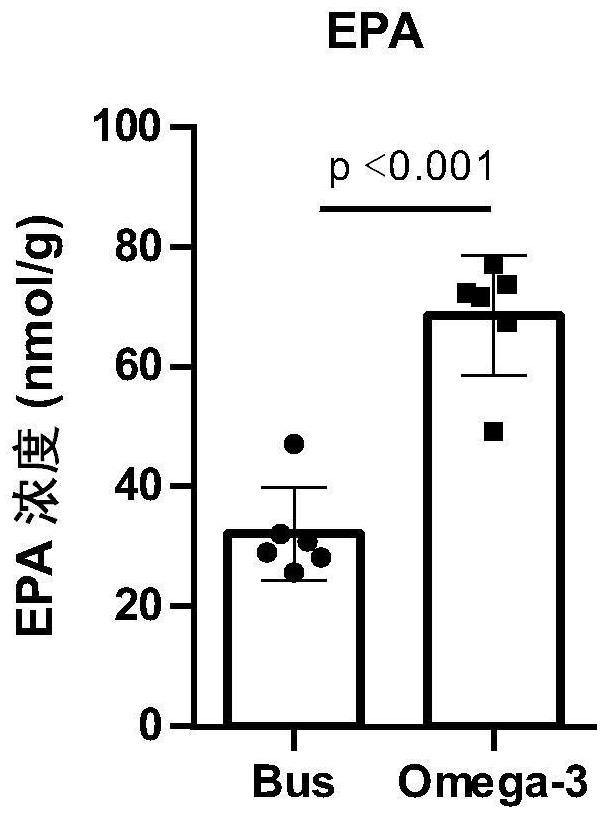
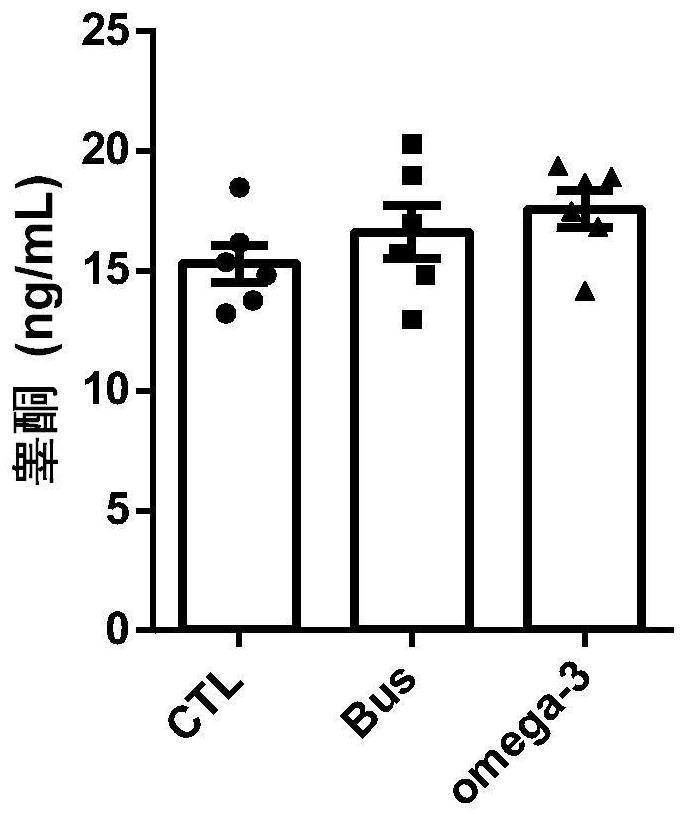
Application of Omega-3 or pharmaceutically acceptable fatty acid thereof to improvement of spermatogenesis disorder
PendingCN114146076APromote proliferationGood treatment effectOrganic active ingredientsSexual disorderMouse TesticlePharmaceutical medicine
The invention discloses a novel application of Omega-3 or pharmaceutically acceptable fatty acid thereof, and the novel application comprises an application of Omega-3 or pharmaceutically acceptable fatty acid thereof in preparation of a medicine for treating NOA. Through eicosanoic acid targeted metabonomics, it is screened that DHA and EPA in testicular tissue of a polyunsaturated fatty acid Omega-3 gavage male mouse are obviously up-regulated. Experiments prove that Omega-3 is injected into the stomach of a busulfan-induced NOA mouse, the thickness and integrity of testis recovery spermatogenic epithelium and the concentration of epididymis tail sperms can be observed, and meanwhile, proliferation and differentiation of spermatogonium and expression of supporting cell paracrine factors are promoted, so that the Omega-3 can be used as a new target for improving the NOA mouse testis spermatogenic dysfunction; a novel method is provided for treating the non-obstructive azoospermia.
Owner:NANJING MEDICAL UNIV +1
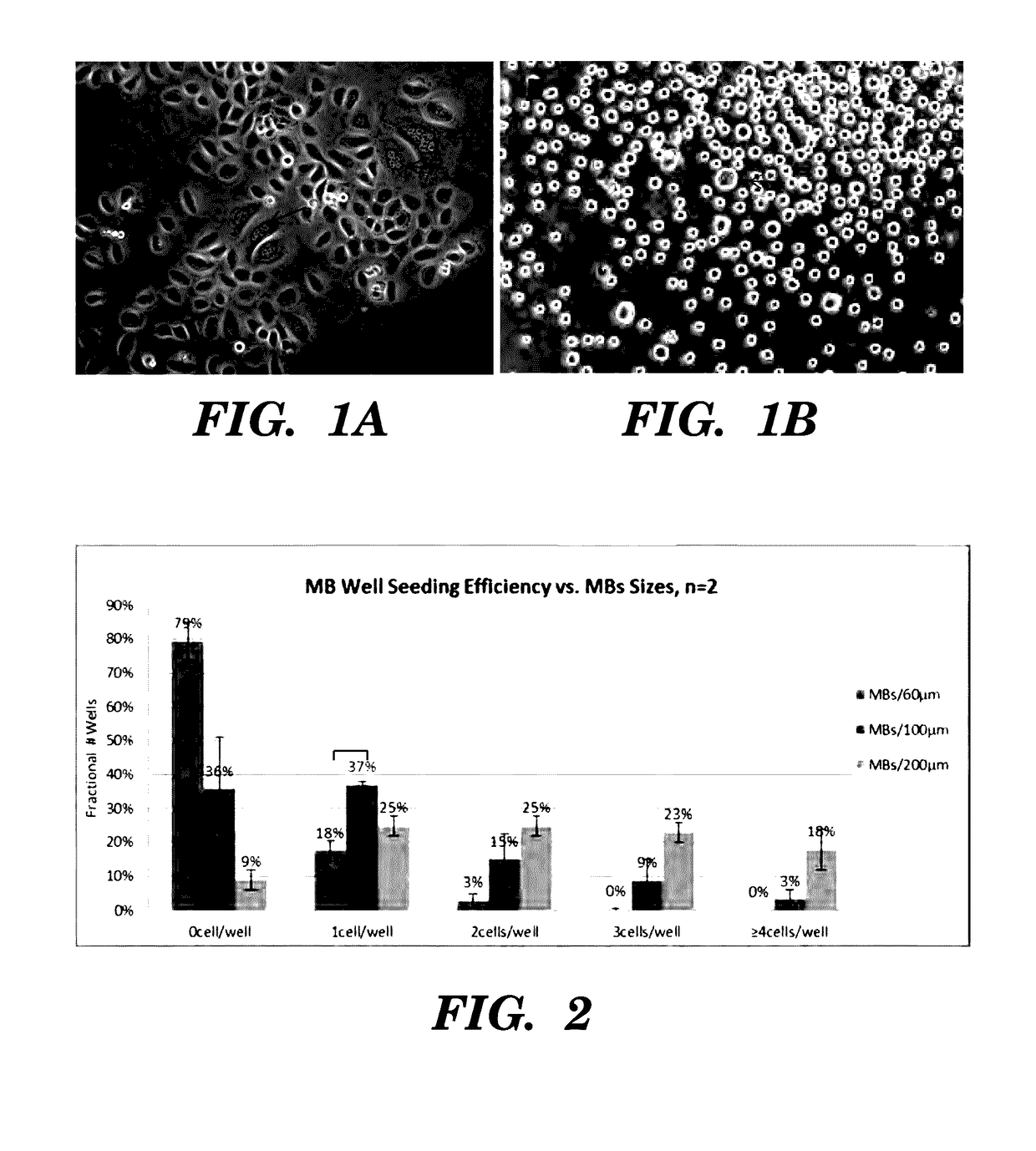
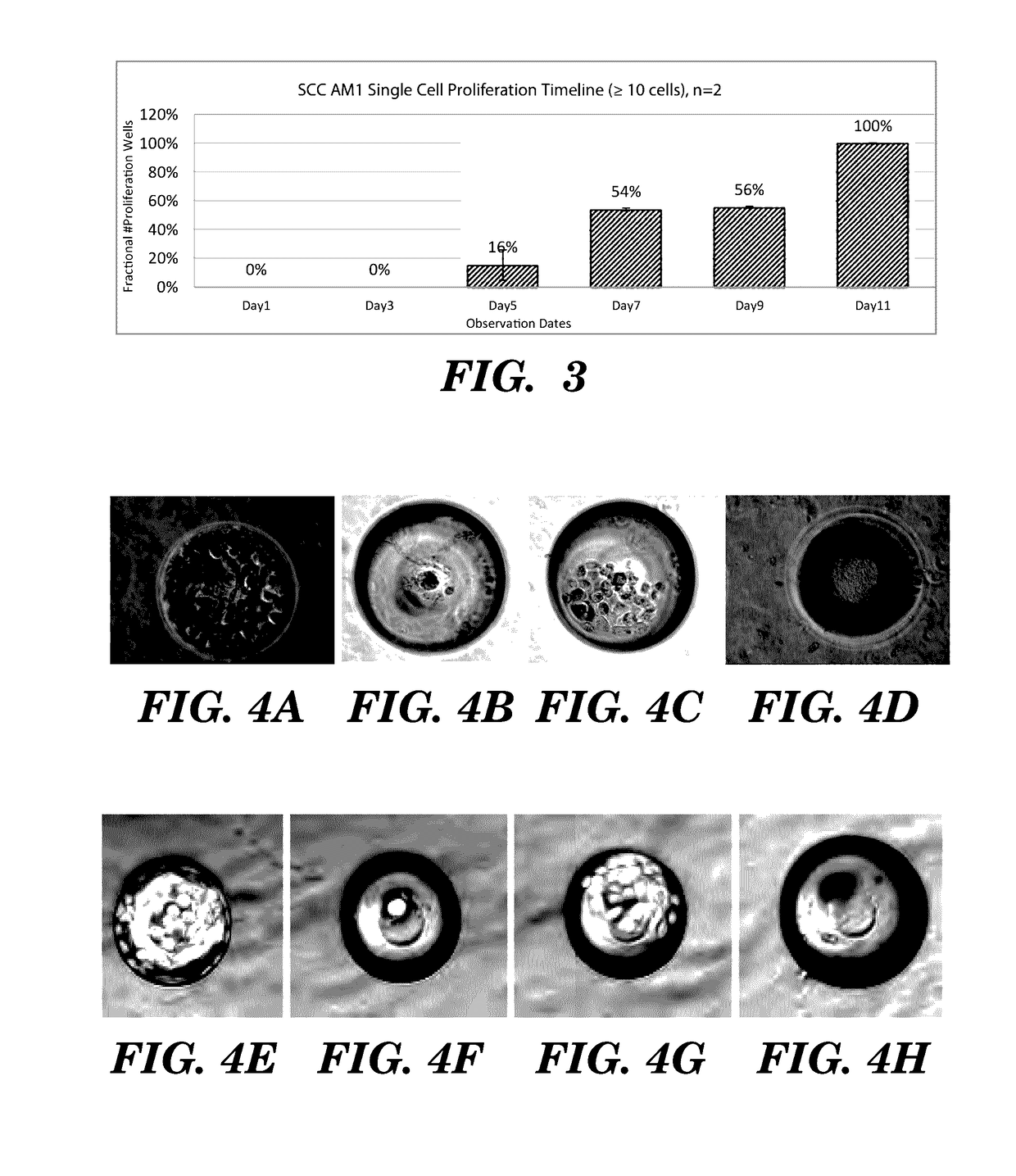
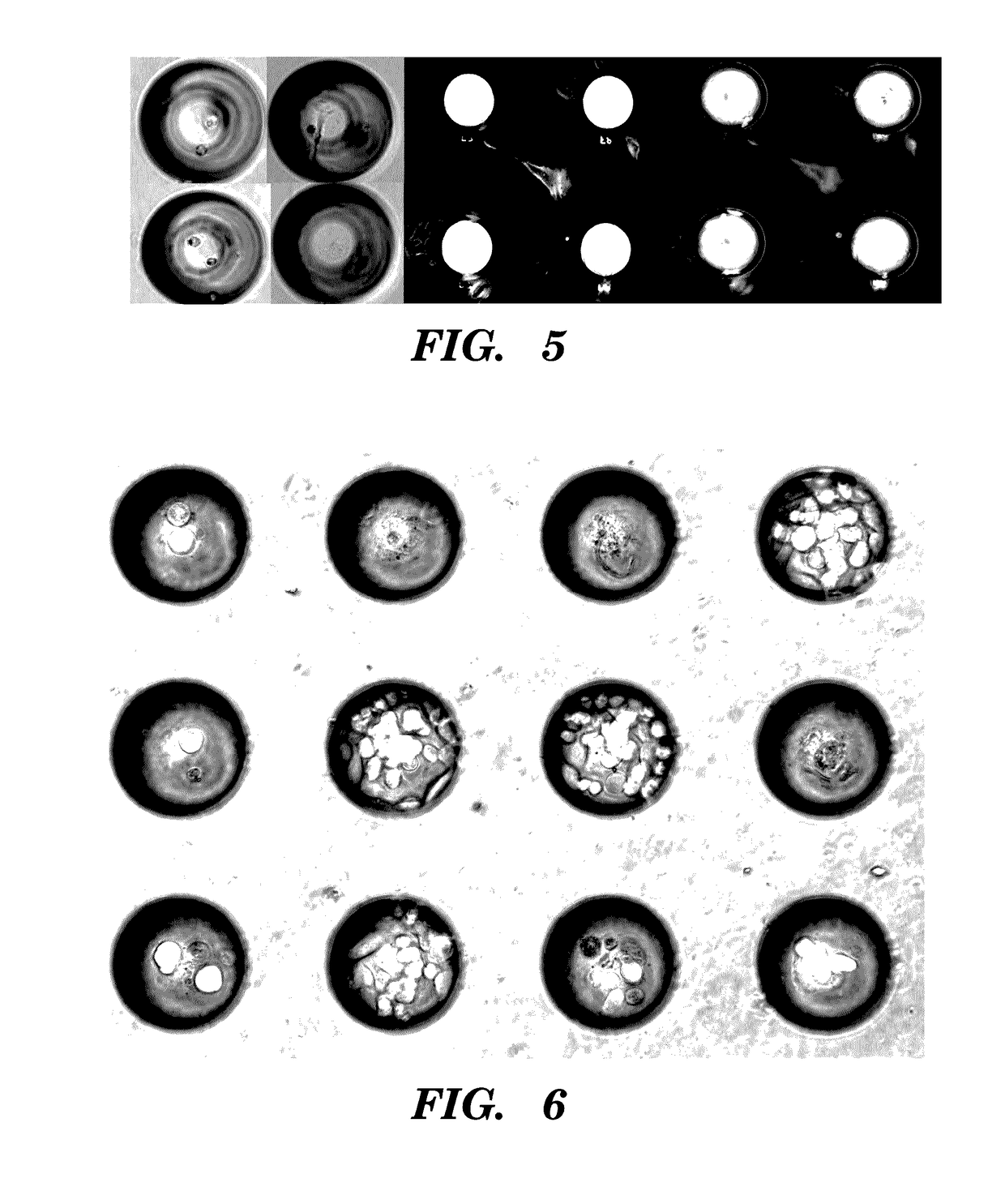
High-throughput cellular analysis using microbubble arrays
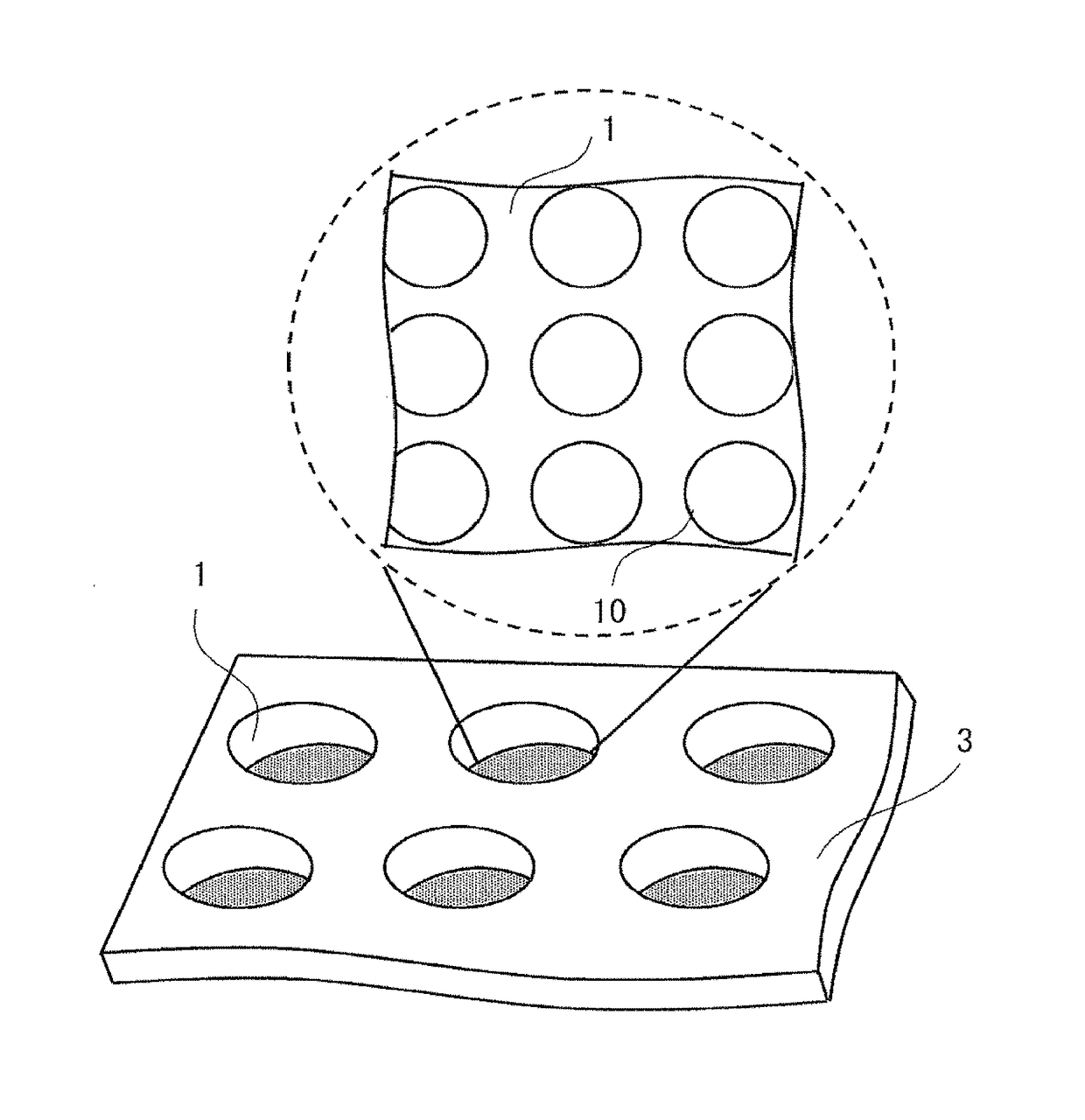
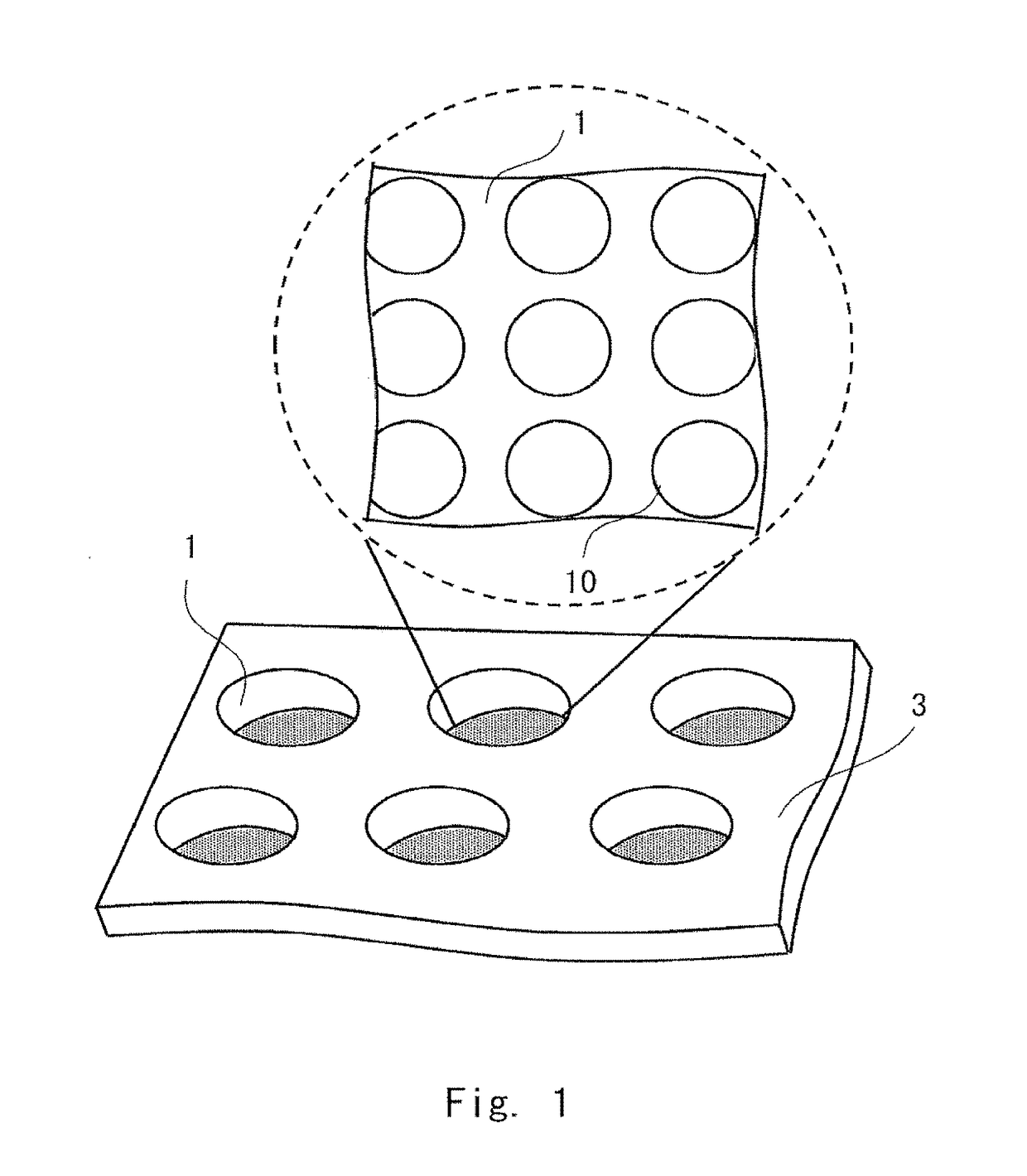
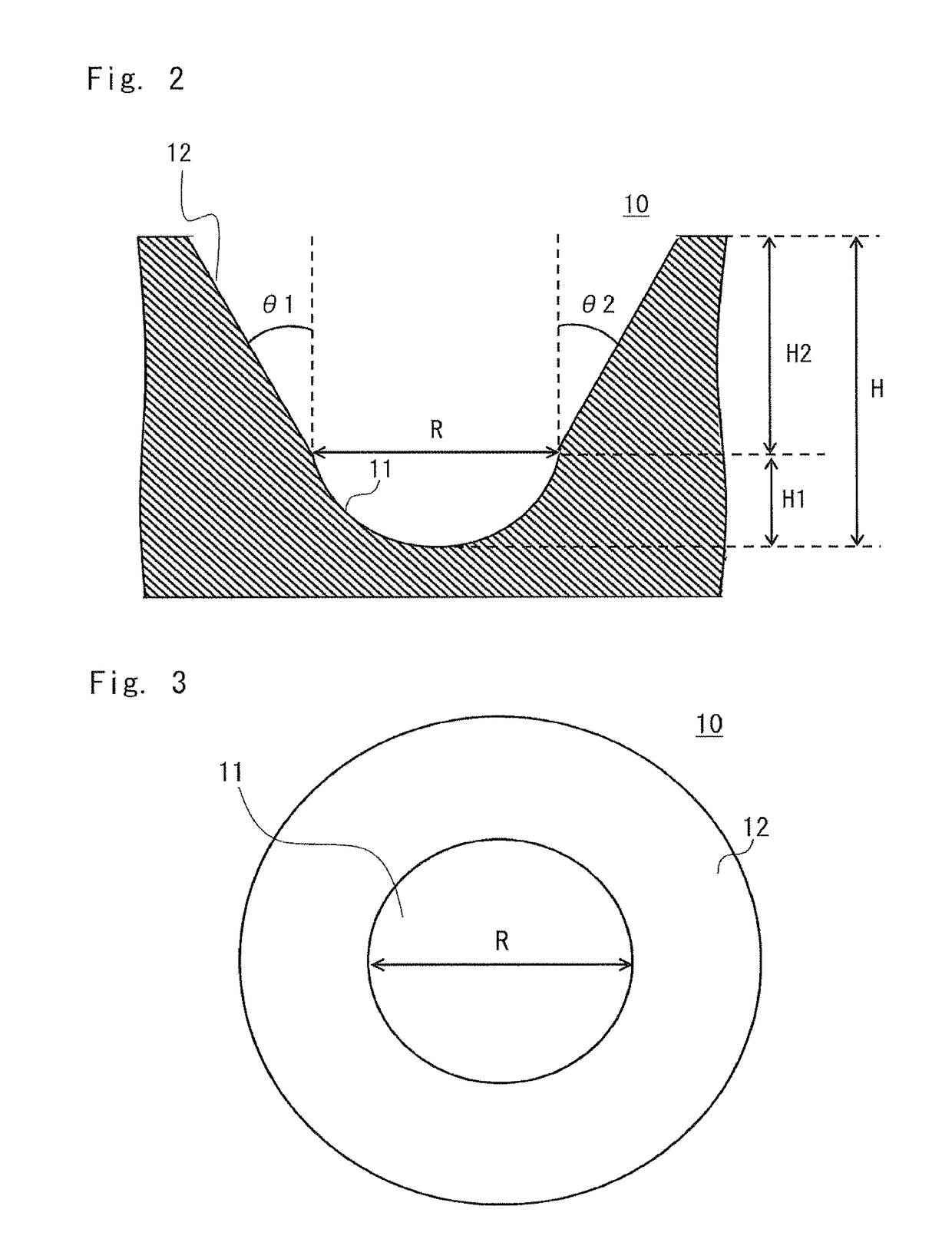
InactiveUS20170067871A1Promote accumulationSimplifying detection and recoveryLaboratory glasswaresMaterial analysisSecretion rateSurface marker
A microfabricated device and method having a substrate with an array of curvilinear cavities that is used for high throughput single cell screening. The substrate of the device is preferably fabricated in a low elastic modulus polymer such as polydimethylsiloxane. The architecture of the cavity forms a small volume micro-niche that seeded cells can rapidly condition to promote survival and proliferation which can be monitored for hours to days to weeks. The cavity architecture allows independent assays to be conducted with minimal influence from nearest neighbor cavities. Methods are disclosed to use the device to, for example, screen single cells by clonal proliferation, clonal morphology, secreted factors, secretion rate, surface markers, and cell functional characteristics including but not limited to migration, drug resistance, the ability to block or promote signaling pathways, or to enhance opsonization.
Owner:UNIVERSITY OF ROCHESTER
Application of vascular secretion factor in preparation of biomarker for detecting non-alcoholic steatohepatitis
ActiveCN114525329APrevent regenerationInhibition induced fibrosisOrganic active ingredientsMetabolism disorderFatty liverHistone deacetylase 2
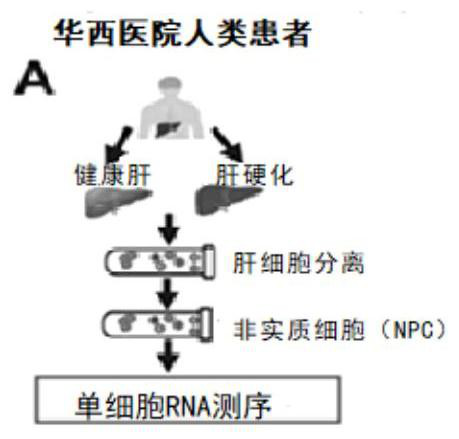
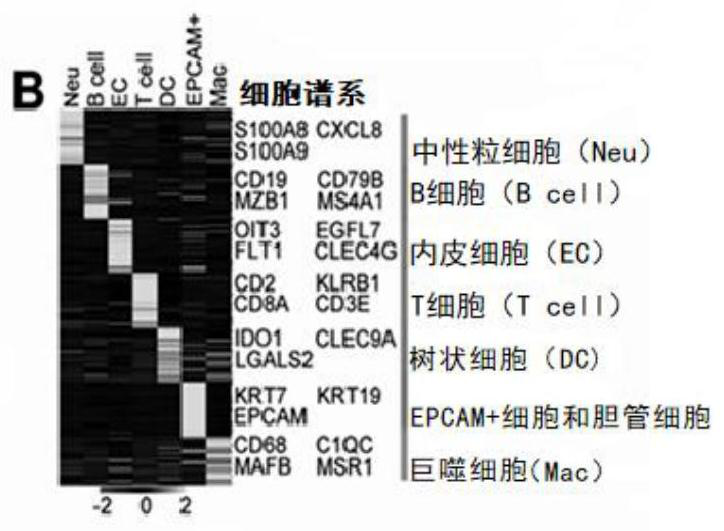
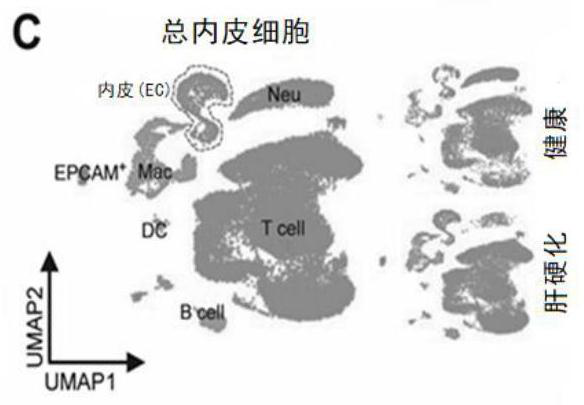
The invention belongs to the field of biological medicine, and mainly relates to application of a vascular secretion factor in preparation of a biomarker for detecting non-alcoholic steatohepatitis. The invention provides an application of a vascular secretion factor in preparation of a biomarker for detecting non-alcoholic steatohepatitis, and is characterized in that the vascular secretion factor comprises IGFBP7 and / or ADAMTS1; the IGFBP7 and / or the ADAMTS1 are / is expressed in an HDAC2 (histone deacetylase 2) / DNMT1-IGFBP7 / ADAMTS1-Th17 signal channel. The invention also provides application of the vascular secretion factor in preparation of the biomarker for distinguishing the non-alcoholic steatohepatitis and the simple fatty liver, and is characterized in that the vascular secretion factor comprises IGFBP7 and / or ADAMTS1; the IGFBP7 and / or the ADAMTS1 are / is expressed in an HDAC2 (histone deacetylase 2) / DNMT1-IGFBP7 / ADAMTS1-Th17 signal channel. The invention further provides application of the synergistic effect of the IGFBP7 and / or the ADAMTS1 in preparation of the biomarker for detecting the non-alcoholic steatohepatitis. According to the application provided by the invention, the non-alcoholic steatohepatitis can be evaluated more reliably, simply, conveniently and noninvasively.
Owner:THE WEST CHINA SECOND UNIV HOSPITAL OF SICHUAN
A kind of anti-hair loss shampoo and preparation method
Owner:山茶元素(北京)科技研发有限公司
Molecular marking method of using neuroendocrine factor genes to select kidding characters
InactiveCN101906480BAccurately estimate breeding valuesImprove selection efficiencyMicrobiological testing/measurementBase JGene selection
The invention discloses a molecular marking method of using neuroendocrine factor genes to select kidding characters. The method comprises the following steps of: using a goat genomic DNA sequence as a template; amplifying Kisspeptin gene intron 2 by using a primer P1 under the PCR condition in the presence of TaqDNA polymerase, buffering environment, Mg2+, dNTPs, and then judging the size of thetarget fragment by agarose gel electrophoresis; detecting the PCR application product of the primer P1 by polyacrylamide gel electrophoresis, finding that the amplification SNPs of the primer P1 has one-base mutation and 8bp-base deletion, and then carrying out genotyping and gene frequency analysis on the amplification product of the primer P1, and the correlation analysis with the kidding characters of Guanzhong diary goat and Xinong Saanen dairy goat. Shown by experiments, the SNPs of the Kisspeptin gene intron 2 detected by the primer P1can be used as molecular marker of the selection of kidding characters.
Owner:NORTHWEST A & F UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com