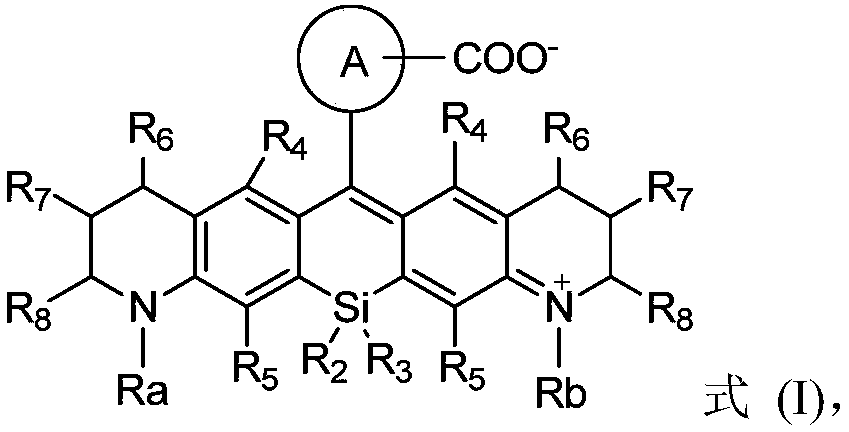
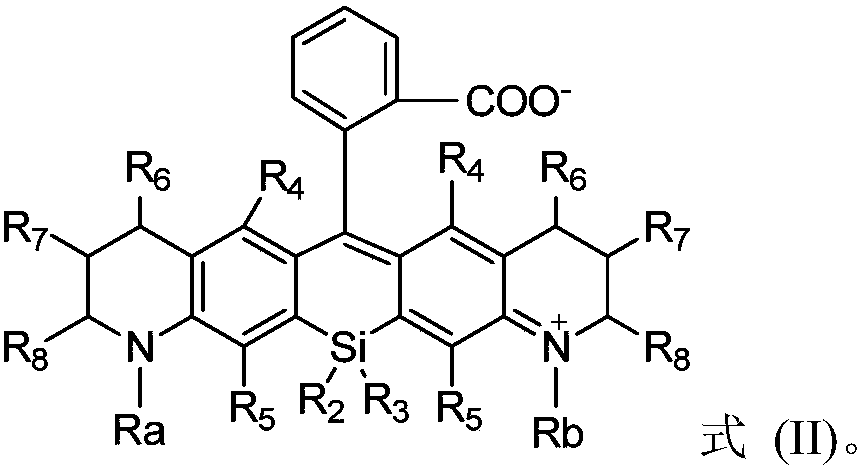
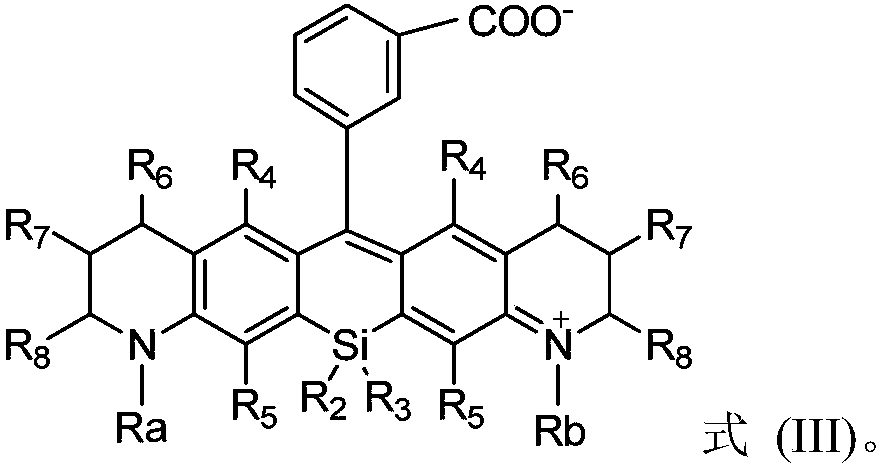
Silicon-based rhodamine derivative and preparation method thereof
An alkyl and alkenyl technology, applied in the field of silyl-based rhodamine derivatives and their preparation, can solve the problems of harsh reaction conditions, many steps, narrow substrate range, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109]
[0110] In a 500 mL round bottom flask equipped with a magnetic stirring bar, 7-bromoquinoline (Aldrich, 2.07 g, 10.0 mmol) and paraformaldehyde (Aldrich, 6.0 g, 200 mmol) were added. Under the protection of argon, anhydrous N,N-dimethylformamide (Aldrich, 20 mL) was added, and magnetically stirred at 0° C. for 10 min. At the same temperature, formic acid (Aldrich, 9.2 g, 200 mmol) was slowly added into the reaction flask. Sodium cyanoborohydride (Aldrich, 6.28 g, 100.0 mmol) was slowly added to the reaction flask in batches. The reaction was slowly warmed to 60°C and stirred overnight. 50 mL of water was added to the reaction solution to quench the reaction, and at 0° C., 3M aqueous sodium hydroxide solution was added to the reaction solution to adjust the reaction solution to alkalinity. Add 100mL of dichloromethane to the reaction solution, separate the organic layer with a separatory funnel, extract the aqueous layer with dichloromethane, combine the organic l...
Embodiment 2
[0112]
[0113] Under argon protection, methylene bridging intermediate (3, 0.31 g, 1 mmol) and anhydrous diethyl ether (aldrich, 10 mL) were added to a 100 mL round bottom flask equipped with a magnetic stirring bar. Stirring for 30 minutes at -78°C, n-BuLi (Aldrich, 1.87mL, 1.6M in n-hexane, 3mmol) was slowly added dropwise to the reaction solution. After the dropwise addition, the reaction was continued at the same temperature for 2 hours. Dimethyldichlorosilane (Aldrich, 0.19 g, 1.5 mmol) was dissolved in 5.0 mL of anhydrous ether, and slowly added dropwise to the above reaction solution. After the dropwise addition was completed, the reaction was slowly raised to room temperature, and the reaction was stirred overnight at room temperature. Add 20 mL of water to the reaction bottle to quench the reaction, add 25 mL of dichloromethane, separate the organic layer, extract the water layer with dichloromethane three times, combine the organic layers, wash once with 20 mL of...
Embodiment 3
[0116] The bromooxazoline reacts with n-butyllithium to generate a lithium reagent, which then undergoes an addition reaction with the key silicone intermediate prepared above. After the addition reaction is completed, hydrochloric acid is added to obtain a silicon-based rhodamine derivative. Its reaction process can be expressed as:
[0117]
[0118] Under argon protection, bromooxazoline derivatives (7, 0.253 g, 1 mmol) and anhydrous diethyl ether (aldrich, 10 mL) were added to a 100 mL round bottom flask equipped with a magnetic stirrer. Stirring for 30 minutes at -78°C, n-BuLi (Aldrich, 1.25mL, 1.6M in n-hexane, 2mmol) was slowly added dropwise to the reaction solution. After the dropwise addition, the reaction was continued at the same temperature for 2 hours. The ketone intermediate (6, 0.376 g, 1 mmol) was dissolved in anhydrous ether (Aldrich, 10 mL), and slowly added dropwise to the aforementioned reaction system at -78°C. After the dropwise addition was complete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com