D-A-D type organic photo-thermal small molecular material and preparation method thereof
A D-A-D, small molecule technology, applied in organic chemistry and other directions, can solve problems such as poor dispersibility and stability, low nanoparticle concentration, and use restrictions, and achieve mild reaction conditions, good dispersibility, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
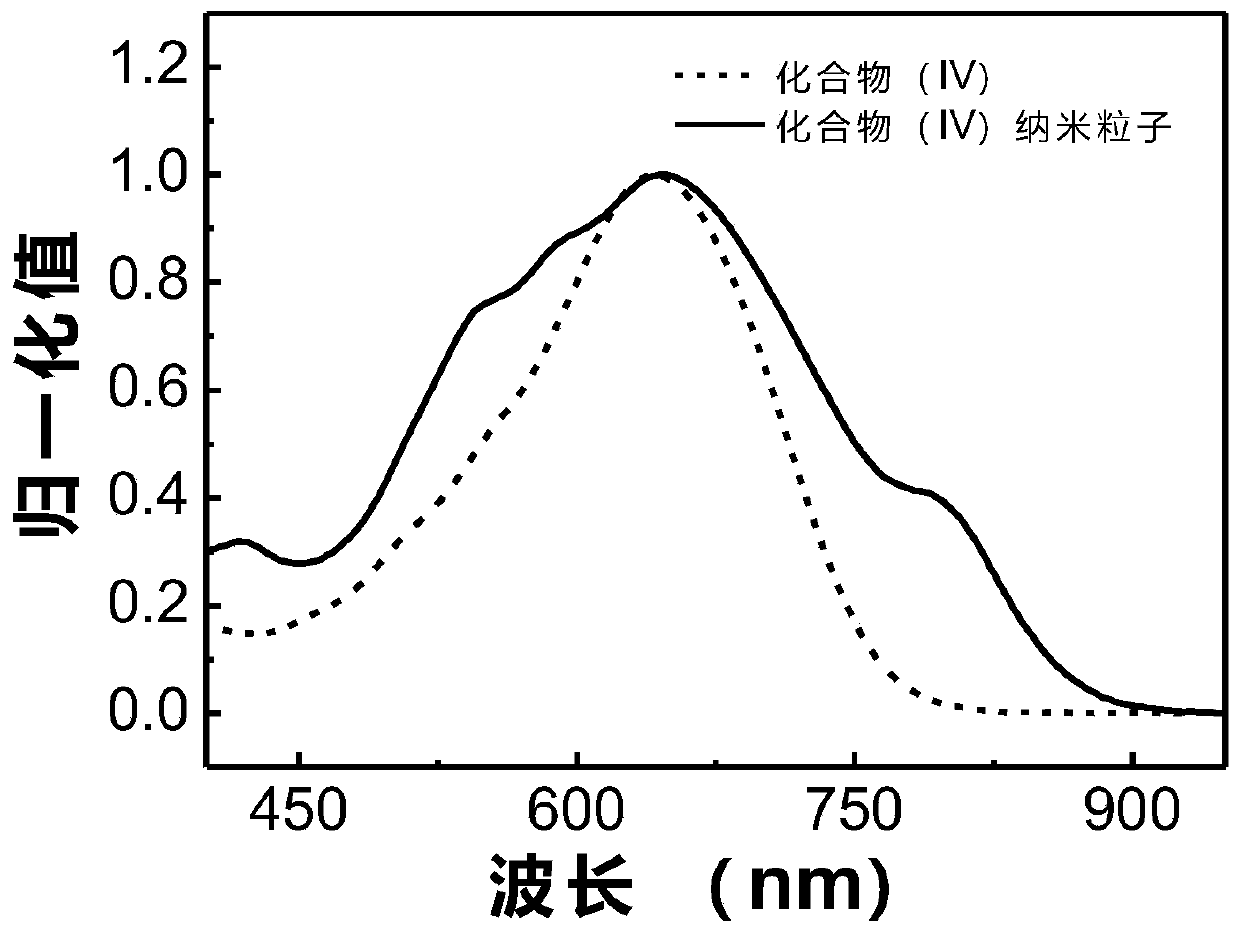
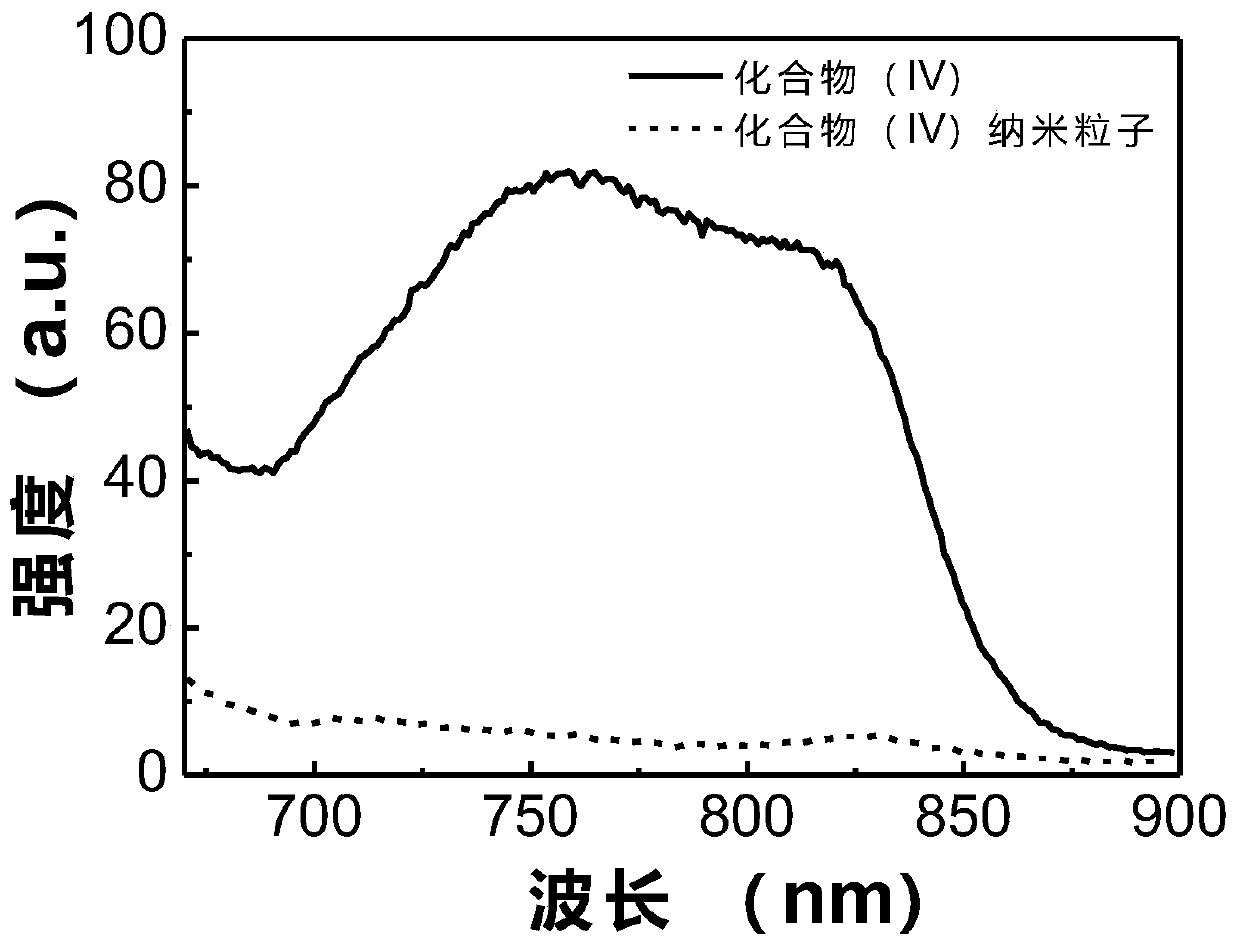
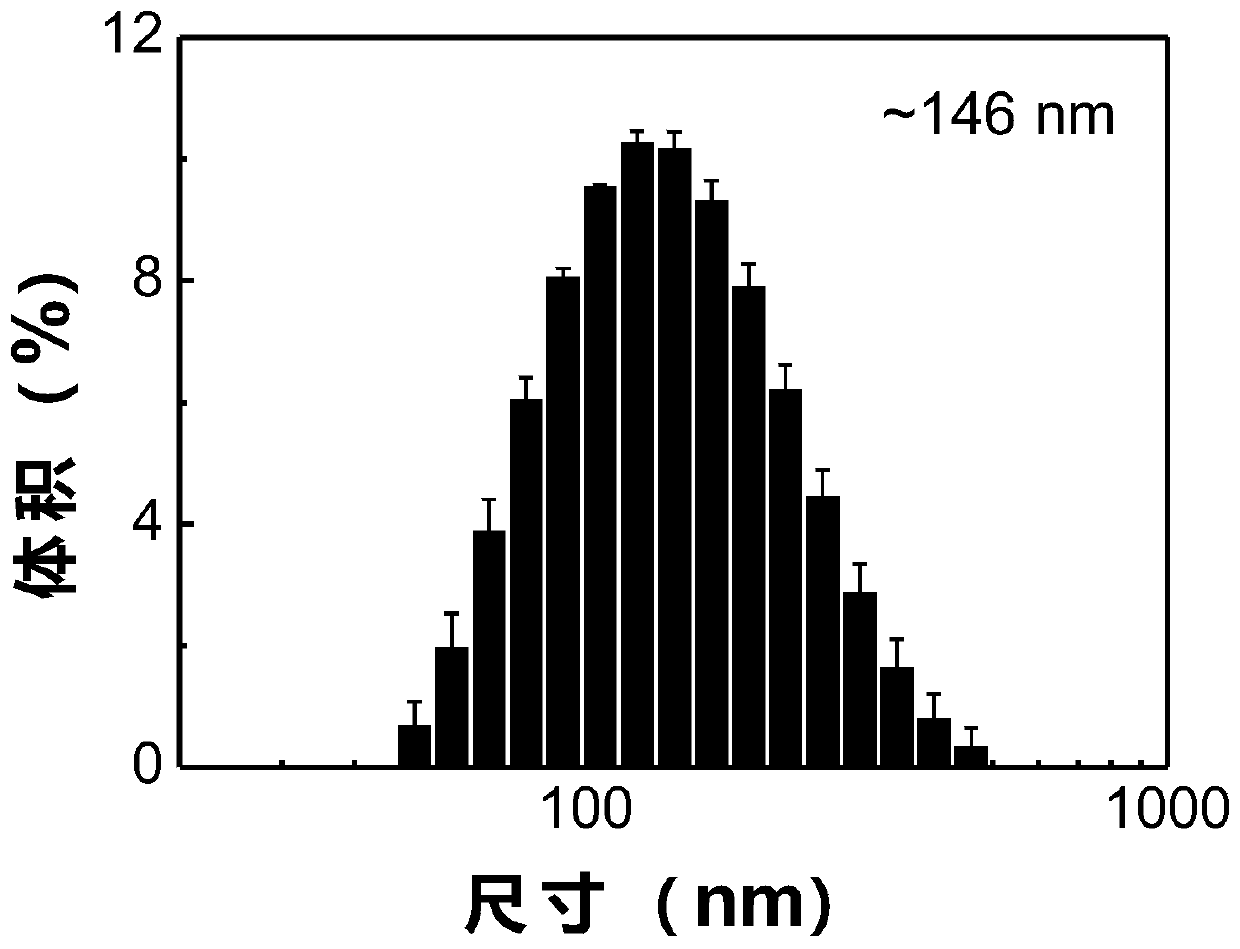
Image
Examples
Embodiment 1
[0031] 1) Add sodium tert-amyloxide (3.00g, 27.2mmol) and 20mL of tert-amyl alcohol to a 250mL three-necked round-bottomed flask. After stirring evenly, add 2-cyanothiophene (2.67g, 24.5mmol), and finally Add diisopropyl succinate (1.66g, 8.2mmol), and react under reflux at 90°C. After reacting for 12h, cool to 60°C, add 30mL of methanol, cool to room temperature, spin to dry the solvent, then water, a small amount of methanol, di After washing with methyl chloride and suction filtration, 2.14 g of pure compound (I) was obtained with a yield of 87.0%. 1 H NMR (DMSO, 600MHz) δ: 8.27(s, 2H), 8.03(d, 2H), 7.35(s, 2H), 3.35(s, 2H);
[0032] 2) Add compound (I) (1.00g, 3.33mmol), bromoisoctane (5.14g, 26.7mmol), cesium carbonate (6.43g, 19.7mmol) in a 250mL three-neck round bottom flask, and then add 50mL of acetonitrile , reflux at 90°C for 15 hours. After the reaction, spin the acetonitrile to dryness, extract with a large amount of water and dichloromethane, spin the organic ph...
Embodiment 2
[0036] 1) Add sodium tert-amylate (0.59g, 5.4mmol) and 5mL of tert-amyl alcohol to a 250mL three-necked round-bottomed flask. After stirring evenly, add 2-cyanothiophene (0.53g, 4.9mmol), and finally Add diisopropyl succinate (0.32g, 1.6mmol), and react under reflux at 90°C. After reacting for 12h, cool to 60°C, add 20mL of methanol, cool to room temperature, spin to dry the solvent, water, a small amount of methanol, di After washing with methyl chloride and suction filtration, 0.35 g of pure compound (I) was obtained with a yield of 72.8%. 1 H NMR (DMSO, 600MHz) δ: 8.27(s, 1H), 8.03(d, 1H), 7.35(s, 2H), 3.35(s, 2H);
[0037] 2) with embodiment 1;
[0038] 3) In a 250mL single-necked round bottom flask, add compound (II) (1.00g, 1.91mmol), then add 70mL of chloroform, and then add N-bromobutyl dissolved in 20mL of chloroform drop by drop with a constant pressure dropping funnel Diimide (0.53g, 2.98mmol), react at room temperature for 3h, stop the reaction, spin the solvent ...
Embodiment 3
[0041] 1) with embodiment 1;
[0042] 2) Add compound (I) (1.00g, 3.33mmol), bromoisoctane (5.14g, 26.6mmol), potassium carbonate (2.72g, 19.7mmol) in a 250mL three-neck round bottom flask, and then add 50mL of acetonitrile , reflux at 90°C for 15 hours. After the reaction, spin the acetonitrile to dryness, extract with a large amount of water and dichloromethane, spin the organic phase to dryness, and then use a mixture of dichloromethane and petroleum ether with a volume ratio of 1:1. column, and the pure product point was collected to obtain 0.55 g of compound (II), with a yield of 31.5%. 1 H NMR (CDCl 3 ,600MHz)δ:8.90(m,2H),7.63(m,2H),7.28(m,2H),4.03(m,4H),1.85(m,2H),1.23-1.42(br,16H),0.84 -1.01(br,12H);
[0043] 3) In a 250mL single-necked round bottom flask, add compound (II) (1.00g, 1.91mmol), then add 70mL of chloroform, and then add N-bromosuccinimide (0.40g, 2.25mmol), react at room temperature for 3h, stop the reaction, spin the solvent to dryness, extract with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com