Fuke'an film coated tablet and preparation process thereof
A technology of film coating and preparation process, which is applied to sugar-coated pills, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Economic benefits, improved product quality, good hydrophobicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
[0024] Embodiment 1~3 prepares sample
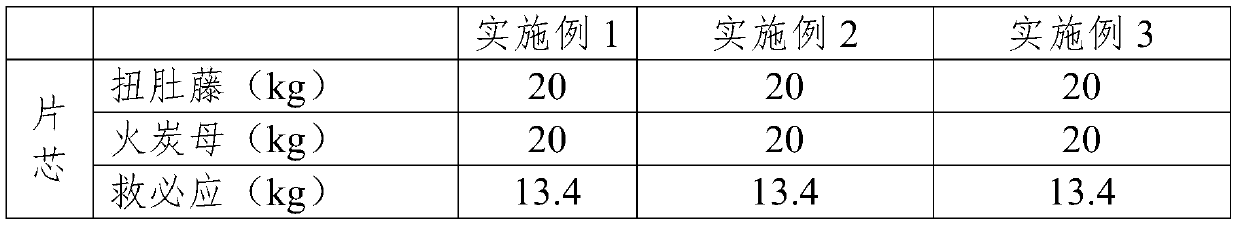
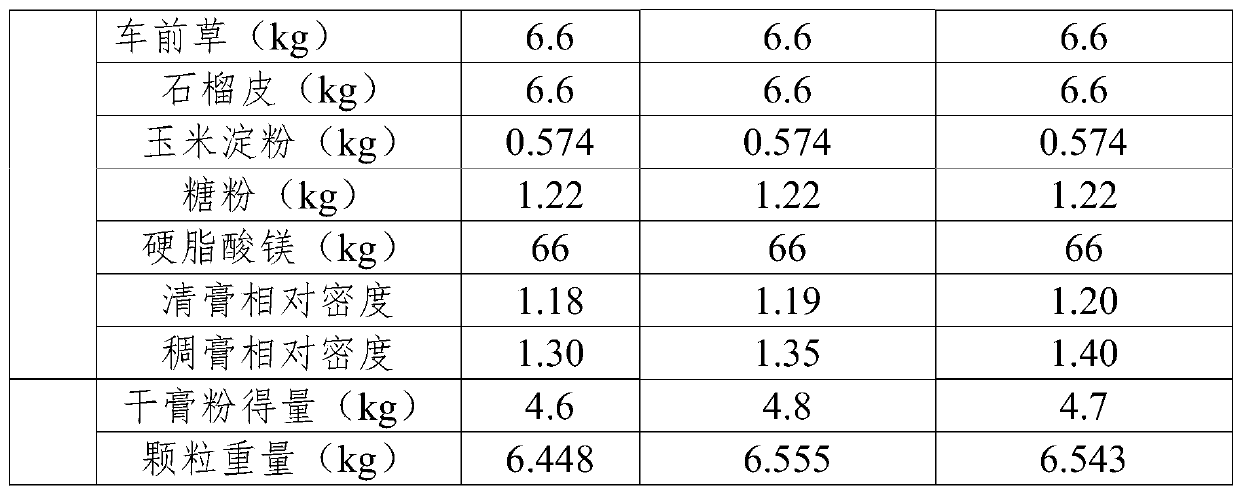
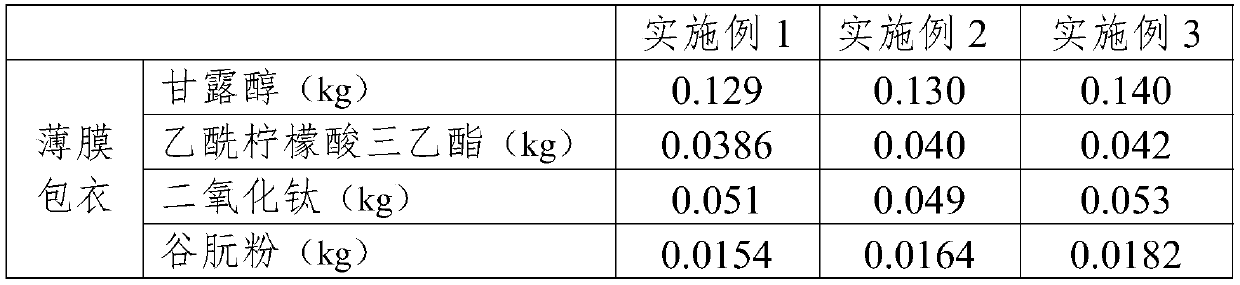
[0025] Embodiments 1-3 provide tablet core prescriptions as shown in Table 1.
[0026]
[0027]
[0028] The tablet core preparation process comprises the following steps:
[0029] (1) Weigh and wash the twig vine, plantain, pomegranate peel, fotan mother, and jiubing, add water to decoct, add 6 times the amount of drinking water, decoct for 2 hours, filter to collect the supernatant, add the filter residue 4 times the amount of drinking water is decocted for the second time for 2 hours, and the 2 extracted medicinal liquids are respectively filtered through 80-mesh sieves, pumped into the medicinal liquid temporary storage tank and combined to obtain medicinal liquid A, and placed at room temperature;
[0030] (2) Pump the medicinal liquid A in the medicinal liquid tank into a two-effect vacuum concentrator, heat and concentrate, measure the specific gravity with a hydrometer at any time, concentrate to a clear ointment with a re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com