Method for catalyzing vitamin A isomerization with ruthenium catalyst
A ruthenium catalyst and isomerization technology, applied in the field of isomerization of vitamin A catalyzed by ruthenium catalysts, can solve the problems of photosensitizer residue, inability to convert cis-isomers, serious environmental pollution, etc., and achieve high isomerization efficiency, Improved recovery rate and low reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Auxiliary preparation:
[0044] (1) Preparation of urea-hydrogen peroxide complex: add 0.708kg 30% hydrogen peroxide (3eq.), 0.0125kg salicylic acid (0.1g / g) and 0.125kg urea (1.0eq.) to a 2L reactor, and keep warm at 5°C reaction. After the reaction is complete, cool down to -5°C, heat and stir for 3 hours, and then centrifuge. The centrifuged solid is rinsed with 0.375L petroleum ether (3ml / g), and the filter cake is a urea-hydrogen peroxide complex with a yield of 80% and a purity of 98%.
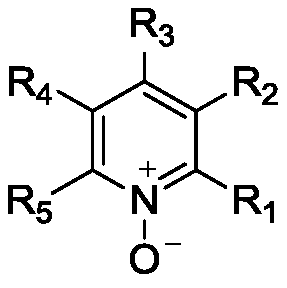
[0045] (2) The reaction process of preparing product 2-methoxypyridine-N-oxide compound: add 0.36L ethyl acetate (3ml / g) in 2L reactor, 0.155kg phthalic anhydride (1.0eq.), 0.109 kg 2-methoxypyridine (1.0 eq.). After the feeding is complete, raise the temperature of the system to 100°C, add 0.20kg of urea-hydrogen peroxide complex (2.0eq) to the system, and keep it warm for reaction; Turn blue again, then adjust the pH of the system to 9 with 20% sodium hydroxide, centrifuge, e...
Embodiment 2
[0051] Auxiliary preparation:
[0052] (1) Preparation of urea-hydrogen peroxide complex: add 0.708kg 30% hydrogen peroxide (3eq.), 0.0125kg salicylic acid (0.1g / g) and 0.125kg urea (1.0eq.) to a 2L reactor, and keep warm at 5°C reaction. After the reaction is complete, cool down to -5°C, heat and stir for 3 hours, and then centrifuge. The centrifuged solid is rinsed with 0.375L petroleum ether (3ml / g), and the filter cake is a urea-hydrogen peroxide complex with a yield of 80% and a purity of 98%.
[0053] (2) The reaction process of preparing the product 2-chloropyridine-N-oxide: add 0.36L ethyl acetate (3ml / g), 0.155kg phthalic anhydride (1.0eq.), 0.12kg to a 2L reactor 2-Chloropyridine (1.0 eq.). After the feeding is complete, raise the temperature of the system to 100°C, add 0.20kg of urea-hydrogen peroxide complex (2.0eq) to the system, and keep it warm for reaction; It turned blue again, then adjusted the pH of the system to 9 with 20% sodium hydroxide, centrifuged, ...
Embodiment 3
[0059] Auxiliary preparation:
[0060] (1) Preparation of urea-hydrogen peroxide complex: add 0.708kg 30% hydrogen peroxide (3eq.), 0.0125kg salicylic acid (0.1g / g) and 0.125kg urea (1.0eq.) to a 2L reactor, and keep warm at 5°C reaction. After the reaction is complete, cool down to -5°C, heat and stir for 3 hours, and then centrifuge. The centrifuged solid is rinsed with 0.375L petroleum ether (3ml / g), and the filter cake is a urea-hydrogen peroxide complex with a yield of 80% and a purity of 98%.
[0061] (2) The reaction process of preparing product 4-picoline-N-oxide: add 0.36L ethyl acetate (3ml / g) in 2L reactor, 0.155kg phthalic anhydride (1.0eq.), 0.093 kg 4-picoline (1.0 eq.). After the feeding is complete, raise the temperature of the system to 100°C, add 0.20kg of urea-hydrogen peroxide complex (2.0eq) to the system, and keep it warm for reaction; Turn blue again, then adjust the pH of the system to 9 with 20% sodium hydroxide, centrifuge, extract, and combine and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com