A kind of purification method of cyclohexene liquid crystal intermediate
A purification method and intermediate technology, which is applied in the field of purification of cyclohexene liquid crystal intermediates, can solve the problems of multi-noble metal catalyst usage, excessive reaction time, affecting product yield and purity, etc., so that the catalyst is not easy to be poisoned and applied mechanically repeatedly , Fewer alkene impurity residues, improved purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
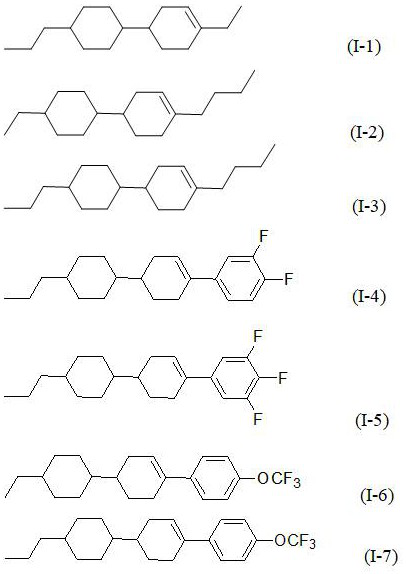
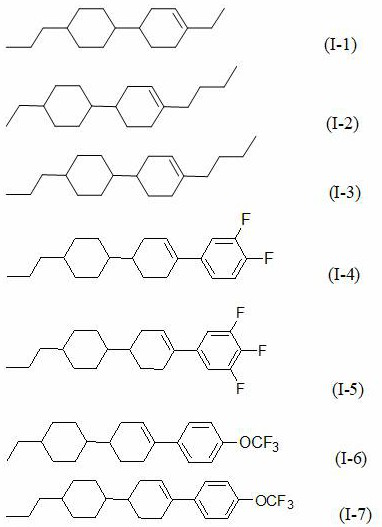
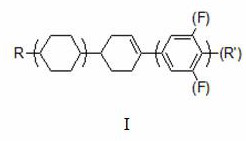
[0032] Add 100g of propylcyclohexylcyclohexenylethane (GC: 99.5%) to the 1L three-necked flask to purify the crude product, add 200ml of non-polar solvent n-hexane, add 25g of active silica gel, add 25g of active powder activated carbon, start stirring, and rotate 50hz, control the temperature of the material system at 60°C, stir and adsorb for 1 hour, and cool down to 25°C; use a filter funnel to filter and separate the solid adsorbent and liquid product, and the liquid product is rotary evaporated to concentrate the solvent. After concentration, add 1 times absolute ethanol to the product and cool down Refrigerate and recrystallize at -30°C for 2 hours, filter out the solid product, and test the product (GC: 99.9%).
[0033] The obtained intermediate after stirring, adsorbing and purifying the mixed adsorbent of silica gel and activated carbon, added 2 times the volume of absolute ethanol, 5% palladium carbon catalyst with 5% weight ratio of the substrate, 0.1MPa hydrogen pre...
Embodiment 2
[0039]Add 100g of propylcyclohexylcyclohexenyl-3,4,5-trifluorobenzene (GC: 99.7%) to a 1L three-neck flask to purify the crude product, add 300ml of non-polar solvent n-hexane, 100ml of toluene, and 25g of active silica gel , add 25g of active powder activated carbon, start stirring, rotate at 50hz, control the temperature of the material system at 60°C, stir and adsorb for 1 hour, then cool down to 25°C; use a filter funnel to filter and separate the solid adsorbent and liquid product, and the liquid product is rotary evaporated to concentrate the solvent and concentrate Finally, 5 times of absolute ethanol was added to the product, cooled to -20°C for 2 hours of freezing and recrystallization, and the solid product was filtered out, and the product was tested (GC: 99.9%).
[0040] After stirring and adsorbing the purified intermediate of silica gel and active carbon mixed adsorbent, add 2 times of volume of absolute ethanol, 3 times of volume of toluene, 5% palladium carbon c...
Embodiment 3
[0046] Add 100g of propylcyclohexylcyclohexenyl-4-trifluoromethoxybenzene (GC: 99.8%) to a 1L three-neck flask to purify the crude product, add 400ml of non-polar solvent n-hexane, add 25g of active silica gel, and add active powder Activated carbon 25g, start stirring, speed 50hz, control material system temperature 60°C, stir and adsorb for 1 hour, cool down to 25°C; use filter funnel to filter and separate solid adsorbent and liquid product, liquid product is rotary evaporated to concentrate solvent, after concentration, add 4 times absolute ethanol, cooled to -20°C, frozen and recrystallized for 2 hours, the solid product was filtered out, and the product was tested (GC: 99.9%).
[0047] After stirring and adsorbing the purified intermediate of silica gel and active carbon mixed adsorbent, add 3 times of volume dehydrated alcohol, 2 times of volume of toluene, 5% palladium carbon catalyst of substrate 5% by weight, 0.1MPa hydrogen pressure, and add Hydrogen reaction for 8 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com