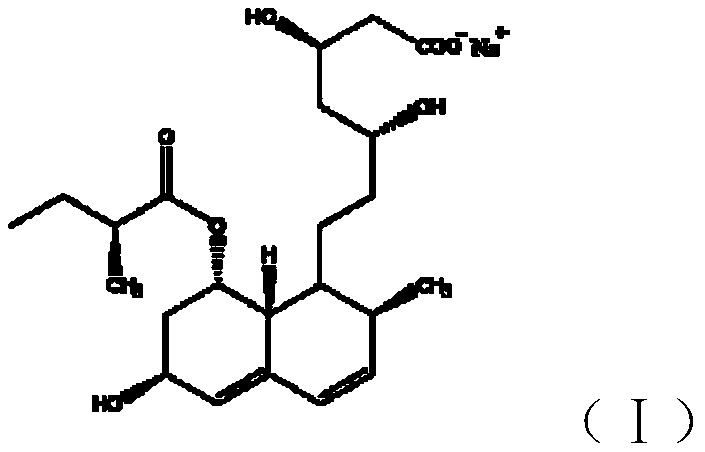
Preparation method for obtaining high-purity crystal form A pravastatin sodium
A technology of pravastatin sodium and pravastatin, which is applied in the field of preparation of high-purity crystal form A pravastatin sodium, can solve the problems of unsuitable drug production, high production cost, and difficult solvent recovery, etc., and facilitate industrial scale promotion Application, low impurity content, stable process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
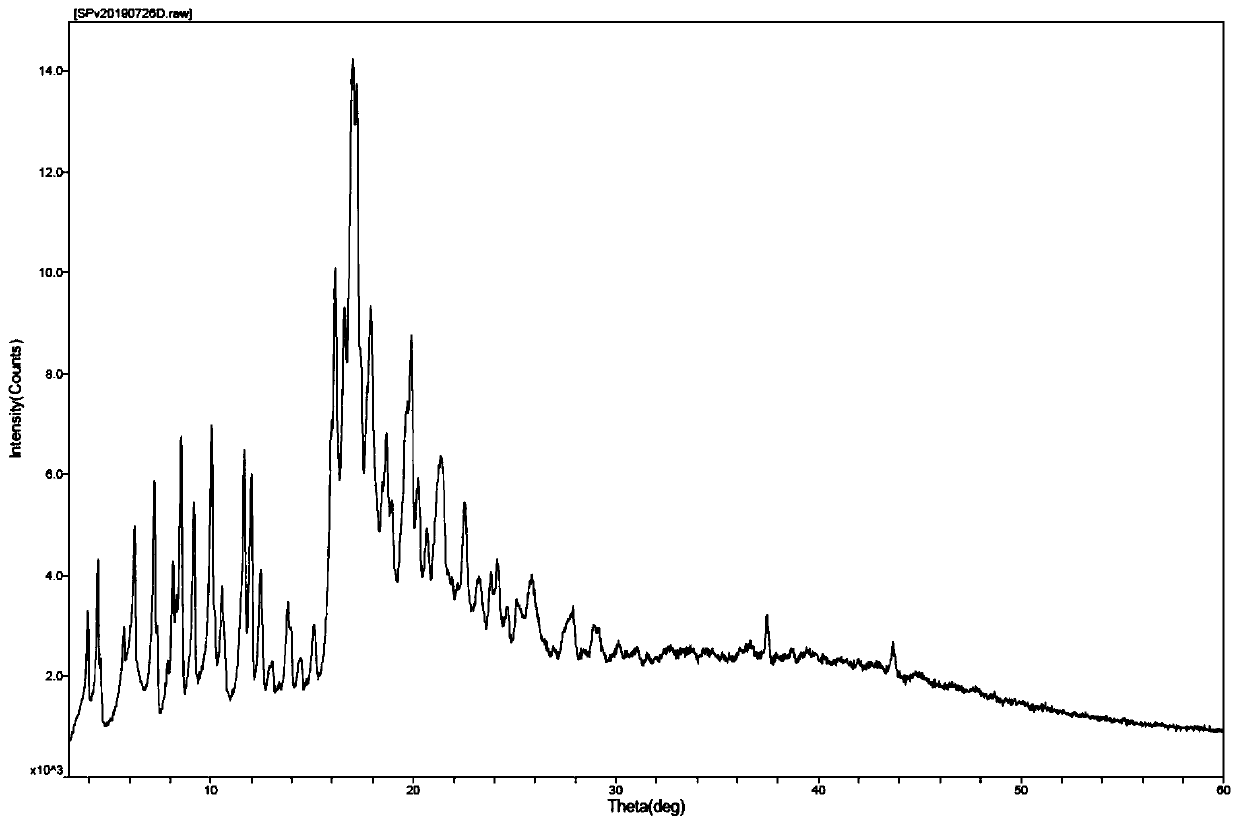
Image
Examples
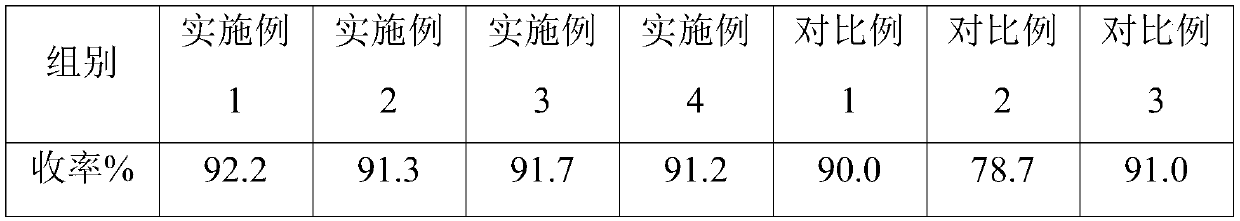
Embodiment 1
[0032] Embodiment 1, a kind of preparation method that obtains high-purity A crystal form pravastatin sodium
[0033] Include the following steps:
[0034] S1) 2300ml of fermented culture fluid containing pravastatin is filtered through a plate frame and the filtrate is collected, the filtrate is 2000ml, adjusted to pH 4.7, extracted with 2000ml of ethyl acetate to obtain the extract;
[0035] S2) Heat the extract in step S1 to reflux for 2h, cool to room temperature, filter with diatomaceous earth, and wash with 500ml, 1% sodium carbonate aqueous solution, the solution after washing is not higher than 50°C and concentrated to 300ml under reduced pressure, and cool down Crystallize at 12°C, filter, and dry under reduced pressure to obtain 22 g of pravastatin lactone crystals;
[0036] S3) Dissolve 22g of pravastatin lactone crystals in step S2 with 220ml of methyl isobutyl ketone at 60°C, add sodium carbonate aqueous solution to wash and stir at room temperature for 1.5h, a...
Embodiment 2
[0039] Embodiment 2, a kind of preparation method that obtains high-purity A crystal form pravastatin sodium
[0040] Include the following steps:
[0041] S1) 2300ml of fermentation broth containing pravastatin was filtered through a plate and frame to collect the filtrate, the filtrate was 2000ml, adjusted to pH 4.5, and extracted with 2000ml of isobutyl acetate to obtain the extract;
[0042] S2) Heat the extract in step S1 to reflux for 1.5h, cool to room temperature, filter with diatomaceous earth, and wash with 500ml, 1.5% sodium carbonate aqueous solution, the solution after washing is not higher than 50°C and concentrated to 300ml under reduced pressure, Cool down to 12°C to crystallize, filter, and dry under reduced pressure to obtain 23 g of pravastatin lactone crystals;
[0043]S3) Dissolve 23 g of pravastatin lactone crystals in step S2 with 345 ml of methyl isobutyl ketone at 50° C., add aqueous sodium carbonate to wash and stir at room temperature for 1.5 h, a...
Embodiment 3
[0046] Embodiment 3, a kind of preparation method that obtains high-purity A crystal form pravastatin sodium
[0047] Include the following steps:
[0048] S1) 2300ml of fermentation broth containing pravastatin was filtered through a plate and frame to collect the filtrate, the filtrate was 2000ml, adjusted to pH 4.5, and extracted with 2000ml of ethyl acetate to obtain the extract;
[0049] S2) Heat the extract in step S1 to reflux for 2h, cool to room temperature, filter with diatomaceous earth, and wash with 500ml, 1% sodium carbonate aqueous solution, the solution after washing is not higher than 50°C and concentrated to 300ml under reduced pressure, and cool down Crystallize at 12°C, filter, and dry under reduced pressure to obtain 22 g of pravastatin lactone crystals;
[0050] S3) Dissolve 22 g of pravastatin lactone crystals in step S2 with 330 ml of methyl isobutyl ketone at 50° C., add aqueous sodium carbonate to wash and stir at room temperature for 2 h, add dilu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap