Degradation-controllable drug-loaded hemostatic sponge and preparation method thereof
A drug-loading and formulation technology, applied in the field of medical materials, can solve the problems of slow degradation and long disappearance time of polyurethane, and achieve simple and accurate clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
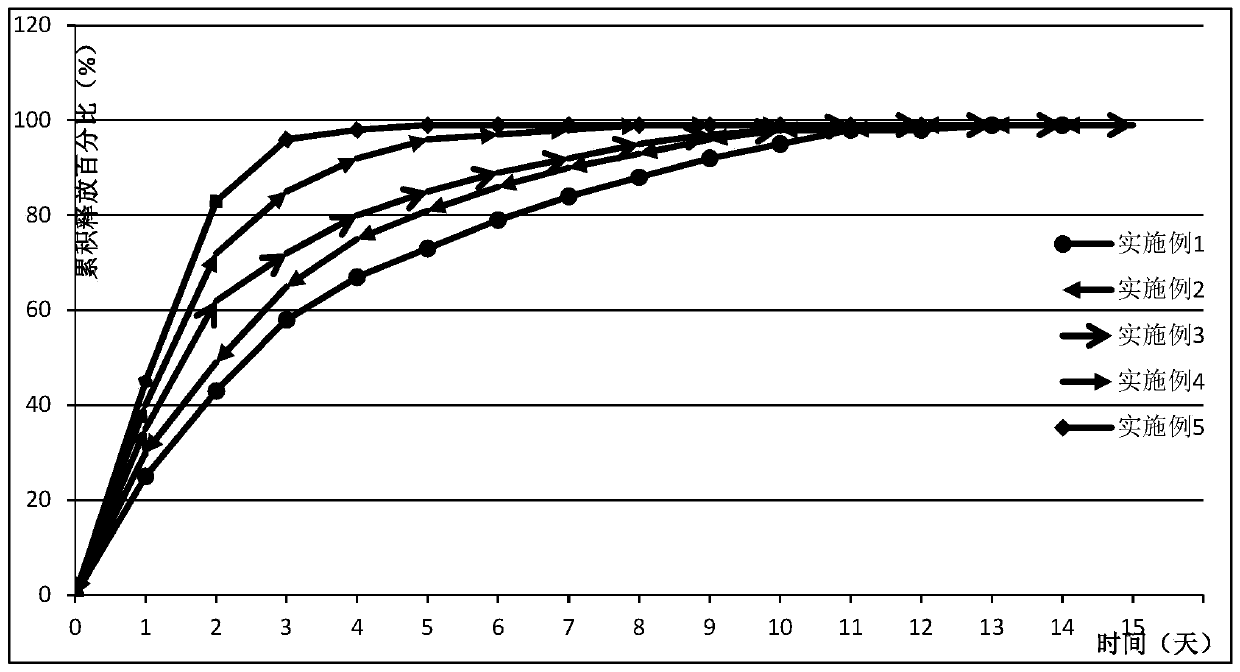
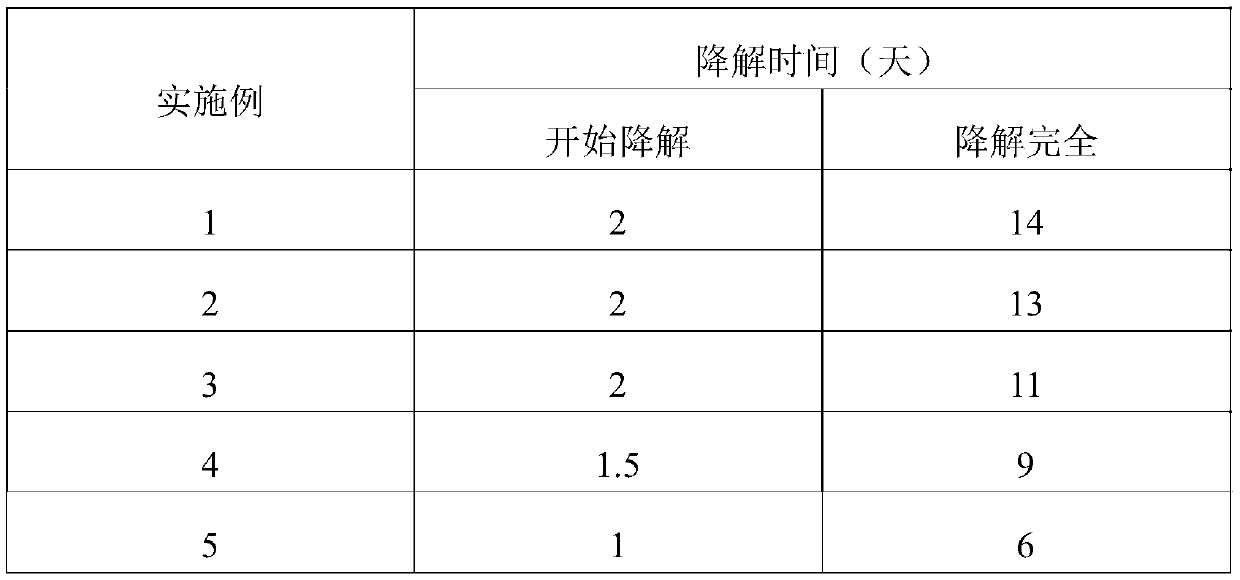
Image
Examples
preparation example Construction
[0029] A method for preparing a drug-loaded hemostatic sponge with controllable degradation, comprising the steps of:
[0030] Step 1, synthesizing DL-lactide and ε-caprolactone prepolymer;
[0031] Under a nitrogen atmosphere, prepare 10-20 parts of DL-lactide and 16-71 parts of ε-caprolactone, and feed DL-lactide and ε-caprolactone with a molar ratio of 10-50:50-90 Mix, add initiator and catalyst respectively, put into an oil bath at 70-140°C and stir for 4-24 hours to obtain DL-lactide and ε-caprolactone prepolymer;
[0032] Step 2, synthetic chain extender;
[0033] Prepare 8-23 parts of diol, 48-138 parts of diisocyanate as raw materials, reaction temperature 60-120 ° C, reaction time 2-6 hours, synthesize chain extender, and purify the product;
[0034] Step 3, preparing a drug-loaded polyurethane sponge precursor solution with controllable degradation;
[0035] Dissolve the prepared DL-lactide and ε-caprolactone prepolymer, the purified chain extender and 0.5-1.5 par...
Embodiment 1
[0040] (1) Synthesis of DL-lactide and ε-caprolactone prepolymer
[0041] Under a nitrogen atmosphere, mix 10gDL-lactide and 71gε-caprolactone (the molar ratio of DL-lactide and ε-caprolactone is 10:90) in a three-necked reaction flask, and then add 56.7g Polyethylene glycol 600 and 324ul stannous octoate were placed in an oil bath at 120°C and stirred for 18 hours to obtain DL-lactide and ε-caprolactone prepolymers;
[0042] (2) Synthetic chain extender
[0043] Weigh 138g of hexamethylene diisocyanate into a three-necked reaction flask, put the reaction flask into an oil bath and heat up to 80°C, then slowly add 23g of 1,4-butanediol dropwise into the reaction system, and continue stirring React for 4 hours. After the reaction, the system was cooled to room temperature, and purified by adding refrigerated n-heptane, and the purified product was characterized by NMR.
[0044](3) Preparation of drug-loaded polyurethane sponge precursor solution
[0045] Weigh 25 g of the d...
Embodiment 2
[0049] (1) Synthesis of DL-lactide and ε-caprolactone prepolymer
[0050] Under a nitrogen atmosphere, mix 10g of DL-lactide and 32g of ε-caprolactone (the molar ratio of DL-lactide and ε-caprolactone is 20:80) in a three-necked reaction flask, and then add 29.4g Polyethylene glycol 600 and 168ul stannous octoate were placed in an oil bath at 130°C and stirred for 14 hours to obtain DL-lactide and ε-caprolactone prepolymers;
[0051] (2) Synthetic chain extender
[0052] Weigh 72g of hexamethylene diisocyanate and add it into a three-necked reaction bottle, put the reaction bottle into an oil bath and raise the temperature to 120°C, then slowly add 12g of 1,4-butanediol dropwise into the reaction system, and continue stirring React for 2 hours. After the reaction, the system was cooled to room temperature, and n-hexane was added for purification, and the purified product was characterized by NMR.
[0053] (3) Preparation of drug-loaded polyurethane sponge precursor solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com