Method for preparing α-hydroxyketone photoinitiator in a microreactor
A photoinitiator and microreactor technology, applied in the field of chemical reaction, can solve the problems of high operating cost, long production cycle, environmental pollution, etc., and achieve the effects of good product quality, short reaction time, and efficient heat and mass transfer capability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
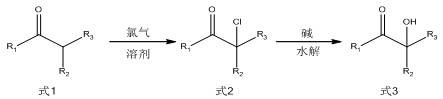
[0037] Example 1: Preparation of 2-hydroxy-2-methylphenylbutanone
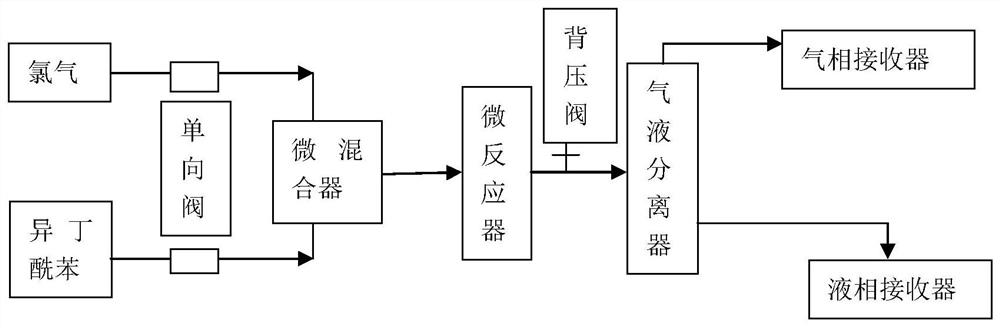
[0038] Isobutyrylbenzene was pumped into a micro-mixer with an inner diameter of 800 μm, and the flow rate of isobutyryl benzene was adjusted to 2 mol / h. The ratio is 1:1.03, isobutyryl benzene and chlorine are mixed in a micro-mixer, the reaction mixture enters a micro-channel reactor with an inner diameter of 800 μm for reaction, the system pressure is 1-2 atm, adjusted by a back pressure valve, and the temperature of the constant temperature water bath is 25 degrees. , the reaction time ends after 30min, the sampling is carried out for gas phase analysis, the reaction selectivity is 99.4%, and the product is directly used for the next step of hydrolysis without purification. The tail gas is the excess chlorine gas and the hydrogen chloride gas produced by the reaction, which is first absorbed by water, mainly absorbs the hydrogen chloride gas produced by the reaction, and then returns to the chlorine gas in...
Embodiment 2
[0040] Example 2: Preparation of 1-hydroxycyclohexyl phenyl ketone
[0041]The dichloroethane solution of cyclohexyl phenyl ketone (the mass ratio of cyclohexyl phenyl ketone and dichloroethane 1:1) was pumped into a micro-mixer with an inner diameter of 800 μm, and the cyclohexyl phenyl ketone was adjusted. The flow rate is 3mol / h, and is pumped into chlorine simultaneously, and the flow rate of adjusting chlorine is 3.18 mol / h, and the ratio of cyclohexyl phenyl ketone and chlorine is 1: 1.06, and cyclohexyl phenyl ketone and chlorine are in micro Mixing in a mixer, the reaction mixture enters a microchannel reaction tube with an inner diameter of 800 μm for reaction, the system pressure is 1-2 atm, the temperature of the constant temperature water bath is 55-60 degrees, and the reaction time ends after 20 minutes. The product was used directly for the next hydrolysis without purification. The tail gas treatment method is the same as that in Example 1.
[0042] The chlorob...
Embodiment 3
[0043] Example 3: Preparation of 2-hydroxy-2-methylphenylbutanone
[0044] Pump the isobutyryl benzene into a micro-mixer with a channel inner diameter of 500 μm, adjust the flow rate of the isobutyryl benzene to 1 mol / h, and pump in the chlorine gas at the same time, adjust the flow rate of the chlorine gas to 1.1 mol / h, the isobutyryl benzene and the chlorine gas The ratio of 1:1.1, isobutyryl benzene and chlorine are mixed in a micro-mixer, the reaction mixture enters a micro-channel reaction tube with an inner diameter of 500 μm for reaction, the system pressure is 1-2 atm, the temperature of the constant temperature water bath is 35 degrees, and the reaction time is 20 minutes later. At the end, sampling was carried out for gas phase analysis, the reaction selectivity was 98.9%, and the product was directly used for the next hydrolysis without purification. The tail gas treatment method is the same as that in Example 1.
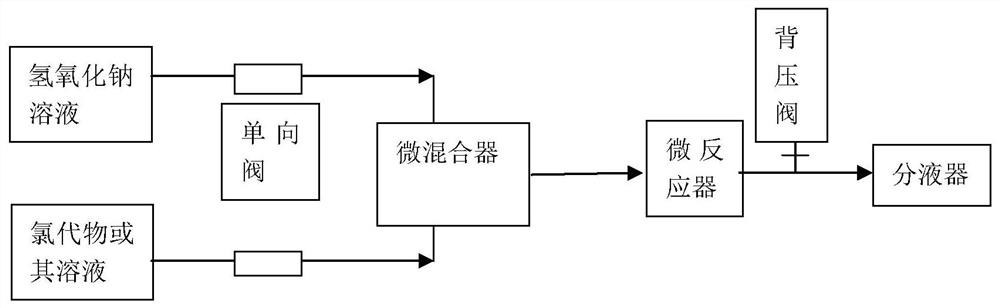
[0045] The above chlorinated product was mixed wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com