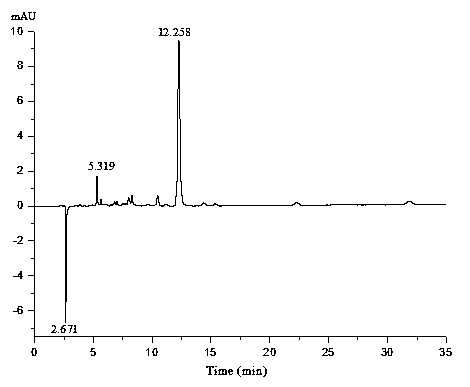
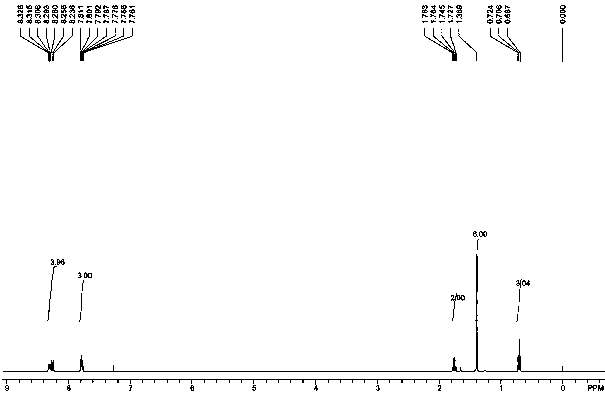
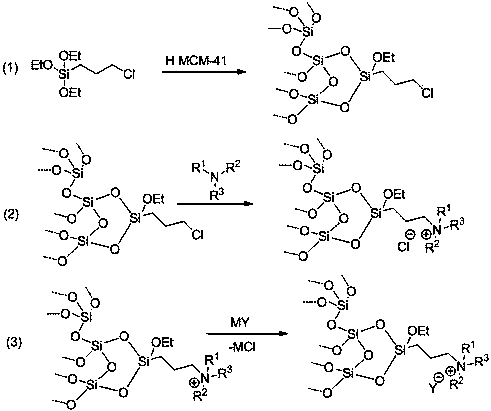
Anthraquinone synthesis catalyst and preparation method thereof
An anthraquinone catalyst and catalyst technology are applied in the preparation of oxidized quinone, chemical instruments and methods, organic compound/hydride/coordination complex catalyst, etc., and can solve the problems of cumbersome operation process, lack of economy, adsorption and the like, To achieve the effect of simple reaction process, avoid corrosion and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The structure of present embodiment catalyst is as follows:
[0038]
[0039] Its preparation method and preparation steps are as follows:
[0040] (1) In a 100 mL reactor, add 50 mL of toluene, add HMCM-41 (3.0 g), chloropropyltriethoxysilane (3.0 g), and stir at 110 °C for 10 h under nitrogen protection , filtered with suction, washed with ethanol (20 mL) three times, and dried in vacuum at 100 °C for 10 h to obtain grafted HMCM-41;
[0041] (2) Using toluene (40 mL) as a solvent, add N,N-diethylbutylamine (1 g) to the grafted HMCM-41 obtained in step (1) and stir at 110 °C for 12 h under nitrogen atmosphere , filtered with suction, washed three times with methanol (20 mL), and dried in vacuum at 100 °C for 10 h to obtain HMCM-41 loaded quaternary ammonium salt.
[0042] (3) Add phosphomolybdenum heteropolyacid (3g) to the slurry after the reaction in step (2), and a metathesis reaction occurs. After a full reaction, the solvent is distilled off, washed with wate...
Embodiment 2
[0048] The structure of present embodiment catalyst is as follows:
[0049]
[0050] Its preparation method and preparation steps are as follows:
[0051] (1) In a 100 mL reactor, add 50 mL of toluene, add HMCM-41 (3.0 g), chloropropyltrimethoxysilane (3 g), and stir at 110°C for 10 h under nitrogen protection. Suction filtration, washing with ethanol (20 mL) three times, and vacuum drying at 100 °C for 10 h to obtain grafted HMCM-41;
[0052] (2) Using toluene (40 mL) as a solvent, add N,N-diethylbutylamine (1 g) to the grafted HMCM-41 obtained in step (1) and stir at 110 °C for 12 h under nitrogen atmosphere , filtered with suction, washed three times with methanol (20 mL), and dried in vacuum at 100 °C for 10 h to obtain HMCM-41 loaded quaternary ammonium salt.
[0053] (3) Add phosphotungstic heteropolyacid (3 g) to the slurry after the reaction in step (2), and a metathesis reaction occurs. After the reaction is complete, the solvent is distilled off, washed with wat...
Embodiment 3
[0058] The structure of present embodiment catalyst is as follows:
[0059]
[0060] Its preparation method and preparation steps are as follows:
[0061] (1) In a 100 mL reactor, add 50 mL of toluene, add HMCM-41 (3.0 g), chlorobutyltriethoxysilane (3 g), and stir at 110 °C for 10 h under nitrogen protection , filtered with suction, washed with ethanol (20 mL) three times, and dried in vacuum at 100 °C for 10 h to obtain grafted HMCM-41;
[0062] (2) Using toluene (40 mL) as a solvent, add N,N-diethylbutylamine (1 g) to the grafted HMCM-41 obtained in step (1) and stir at 110 °C for 12 h under nitrogen atmosphere , filtered with suction, washed three times with methanol (20 mL), and dried in vacuum at 100 °C for 10 h to obtain HMCM-41 loaded quaternary ammonium salt.
[0063] (3) Add cobalt tungsten heteropolyacid (3 g) to the slurry after the reaction in step (2), and a metathesis reaction occurs. After sufficient reaction, the solvent is distilled off, washed with wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com