Method for recycling cobalt and lithium in cathode material of waste lithium cobalt oxide battery by using deep eutectic solvent
A low eutectic solvent, battery cathode technology, applied in the direction of improving process efficiency, can solve problems such as easy generation of toxic gas, secondary pollution, unfavorable environmental protection, etc., and achieve the effects of being beneficial to environmental protection, less dangerous, and easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
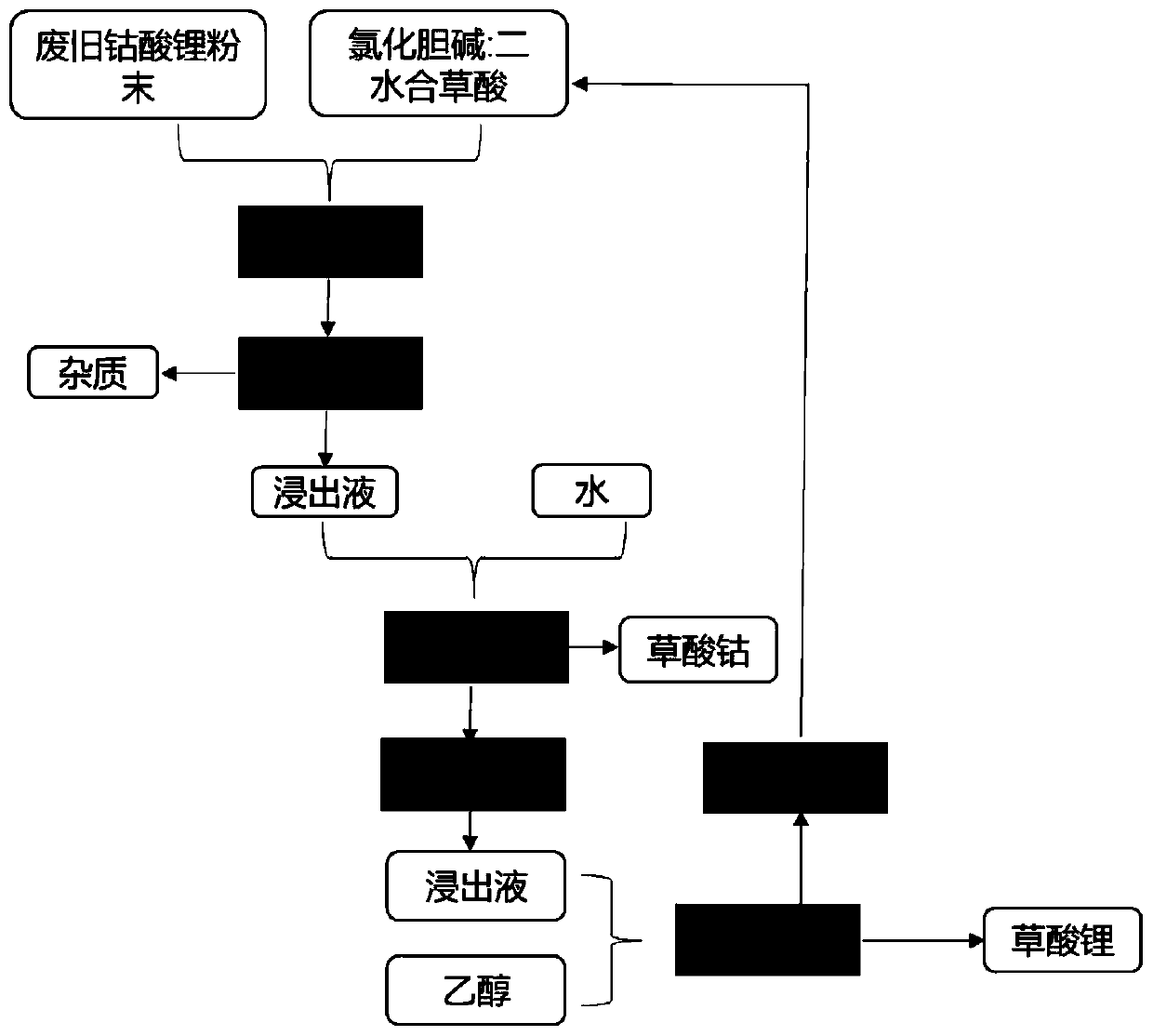
[0027] (1) Choline chloride and oxalic acid dihydrate are mixed in a 1:1 molar ratio to form a deep eutectic solvent;
[0028] (2) Mix 5 mL of the deep eutectic solvent obtained in step (1) with 0.1 g of waste lithium cobalt oxide battery positive electrode material powder, and heat and leach at 90°C for 1 hour, and obtain insoluble impurities and leachate after solid-liquid separation;
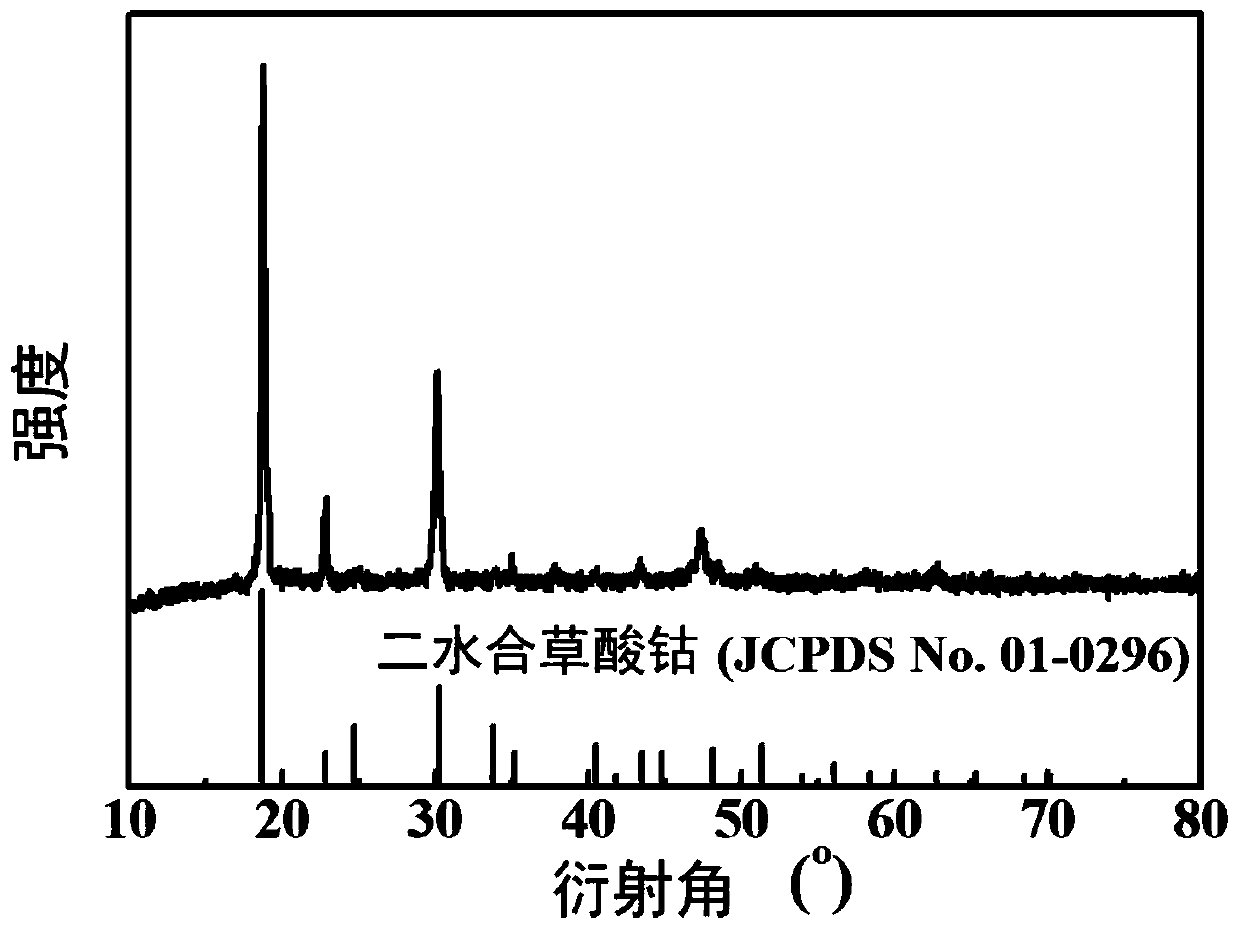
[0029] (3) adding deionized water to the leachate obtained in step (2) to obtain a cobalt oxalate precipitate, separating the precipitate and the leachate, drying the precipitate to obtain a cobalt oxalate solid, and the recovery rate of the cobalt element reaching 93%;
[0030] (4) Heating and concentrating the leaching solution to remove deionized water, adding ethanol to obtain lithium oxalate precipitation, separating the precipitation and leaching solution, drying the precipitation to obtain lithium oxalate solid, and the recovery rate of lithium element reaches 85%.
Embodiment 2
[0032] (1) Choline chloride and oxalic acid dihydrate are mixed in a 1:1 molar ratio to form a deep eutectic solvent;
[0033] (2) Mix 5mL of the deep eutectic solvent obtained in step (1) with 0.2g of waste lithium cobaltate battery positive electrode material powder, and heat and leach at 90°C for 80min, and obtain insoluble impurities and leachate after solid-liquid separation;
[0034] (3) adding deionized water to the leachate obtained in step (2) to obtain a cobalt oxalate precipitate, separating the precipitate and the leachate, drying the precipitate to obtain a cobalt oxalate solid, and the recovery rate of the cobalt element reaching 90%;
[0035] (4) Heating and concentrating the leaching solution to remove deionized water, adding ethanol to obtain lithium oxalate precipitation, separating the precipitation and leaching solution, drying the precipitation to obtain lithium oxalate solid, and the recovery rate of lithium element reaches 83%.
Embodiment 3
[0037] (1) Choline chloride and oxalic acid dihydrate are mixed in a 2:1 molar ratio to form a deep eutectic solvent;
[0038] (2) Mix 5 mL of the deep eutectic solvent obtained in step (1) with 0.1 g of waste lithium cobaltate cathode material powder, and leaching at 90°C for 80 min, and obtain insoluble impurities and leachate after solid-liquid separation;
[0039] (3) adding deionized water to the leachate obtained in step (2) to obtain a cobalt oxalate precipitate, separating the precipitate and the leachate, drying the precipitate to obtain a cobalt oxalate solid, and the recovery rate of the cobalt element reaching 88%;
[0040] (4) Heating and concentrating the leaching solution to remove deionized water, adding ethanol to obtain lithium oxalate precipitation, separating the precipitation and leaching solution, drying the precipitation to obtain lithium oxalate solid, and the recovery rate of lithium element reaches 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com