A kind of antibacterial peptide and its preparation method and application
An antibacterial peptide, peptide amino acid technology, applied in antibacterial drugs, peptides, pharmaceutical formulations and other directions, can solve the problems of high cost, low yield, limitations, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: Fermentation obtains the thalline containing expression recombinant protein
[0048] Construction of recombinant genetic engineering bacteria BL pET30a ABP-1 of antibacterial peptide ABP-1:
[0049] The amino acid sequence of the antimicrobial peptide ABP-1 is:
[0050] HHHHHHSSSSGSSSS(DDDDKYYEERRWQWRGSGRWQWRRFFFKKRSSGSS) 10 Among them, HHHHHH is the nickel chelation column purification label; SSSSGSSSS and SSGSS are transition peptides, and DDDDK is the cleavage site of enterokinase.
[0051] According to the codon preference of Escherichia coli, the nucleotide sequence encoding the above antibacterial peptide ABP-1 is artificially synthesized as shown in SEQID NO.2. Among them, catatg and ggtacc are restriction endonucleases NdeI and KpnI enzyme cutting sites.
[0052] After the nucleotide sequence was digested with restriction endonucleases NdeI and KpnI, it was inserted into the NdeI and KpnI of the multiple cloning site of the Escherichia coli expr...
Embodiment 2
[0070] Embodiment 2: Purification to target protein
[0071] After the IPTG-induced expression of the bacterial cells obtained in step 4 of Example 1, the bacterial cells collected by centrifugation were ultrasonically lysed, and the inclusion body precipitates were collected by centrifugation. After the inclusion bodies were washed with washing solution (50mmol / L phosphate buffer, 1% Triton 100, 2mol / L urea, pH7.8), centrifuge at 10000r / min for 20min to collect the inclusion body precipitates, and then use the extraction solution (50mmol / L phosphate buffer, 8mol / L urea and 2mmol / L DTT, pH7.8) after fully dissolved, pass through an ultrafiltration membrane with a molecular weight of 50,000 to collect the filtrate for later use.
[0072] Nickel Sepharose FF or Nickel NTA Sepharose FF prepacked column, equilibrate to baseline with equilibration buffer, and equilibrate to baseline with 8M urea-2mmol / L DTT-equilibrium buffer pH7.8.
[0073] Take the above ultrafiltrate and load t...
Embodiment 3
[0077] The antimicrobial peptide ABP-1 sample prepared in Example 2 was inserted into LB medium for filtration and sterilization to prepare a 2000 μg / mL antimicrobial peptide mother solution. Then it was diluted to obtain 1000 μg / mL and 200 μg / mL antimicrobial peptide solutions respectively.
[0078] The antimicrobial peptide solution prepared above was transferred to cultured Escherichia coli ATCC 25922, Bacillus subtilis ATCC9372, Salmonella ATCC14028, Pseudomonas aeruginosa ATCC27853, and Staphylococcus aureus ATCC25923.
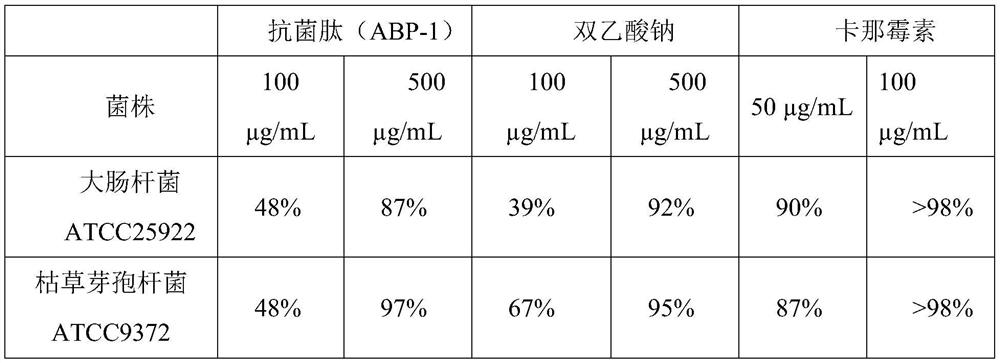
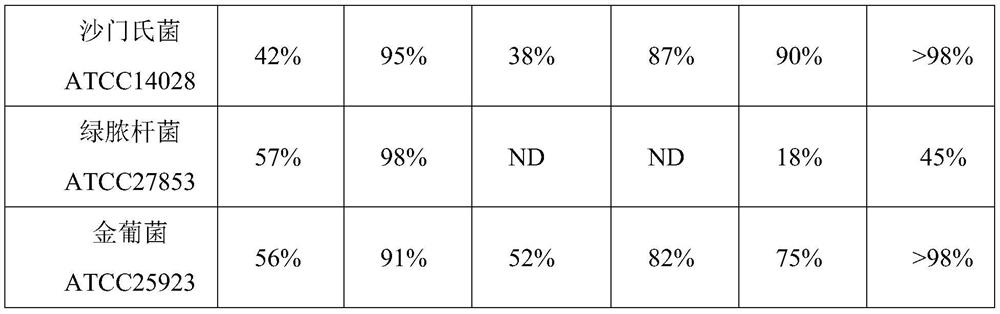
[0079] Specifically, PCR tubes were taken and grouped according to different concentrations of antimicrobial peptide (ABP-1), target bacteria inhibited, sodium diacetate control and kana control, see Table 1 for details. Add 100 μL prepared different concentrations of antibacterial peptide ABP-1 and 100 μL prepared Escherichia coli ATCC 25922, Bacillus subtilis ATCC9372, Salmonella ATCC14028, Pseudomonas aeruginosa ATCC27853, Staphylococcus aureus ATCC259...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com