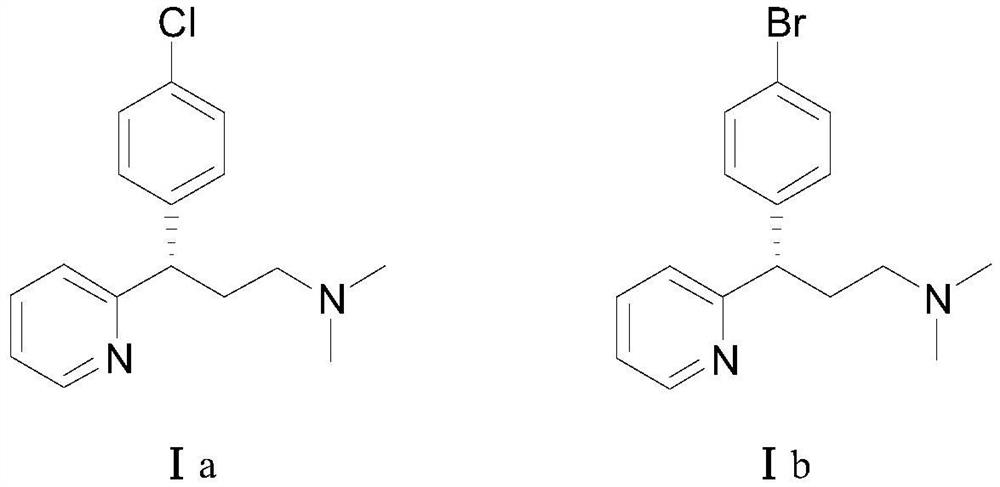
A kind of asymmetric synthesis method of dexchlorpheniramine and dexbrompheniramine
A synthetic method, the technology of D-halogenated pheniramine, applied in the field of chemical synthesis, can solve the problems of a large number of split reagents and poor atom economy, and achieve high industrial production value, short route, high yield and good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]
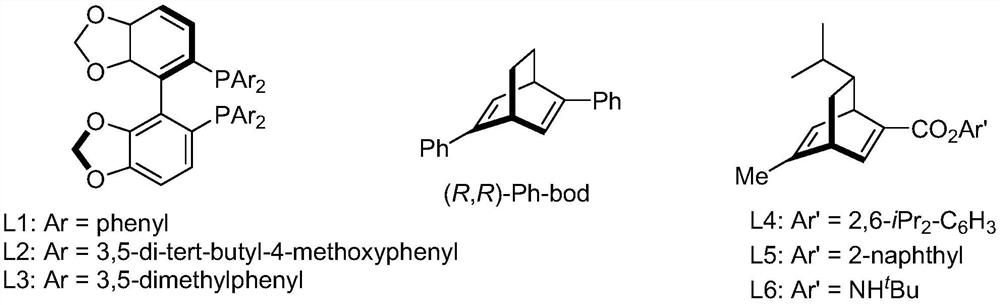
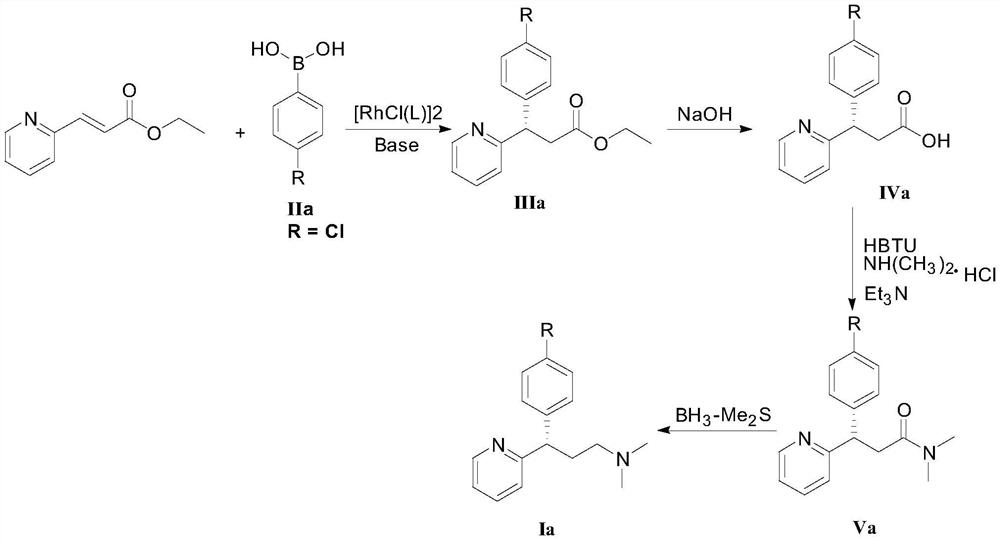
[0048] Ethyl 3-(2-pyridyl)acrylate (35.4 mg, 0.20 mmol), 4-chlorophenylboronic acid (compound IIa, 48.8 mg, 0.40 mmol), chiral rhodium catalyst [RhCl(L3)] 2(1.5 mg, 0.20 mmol%, that is, the molar weight of the chiral rhodium catalyst is 0.20% of the molar weight of 3-(2-pyridyl) ethyl acrylate) was added to the Schlenk tube, and tetrahydrofuran (1 mL) was sequentially added under the protection of high-purity nitrogen. And potassium hydroxide aqueous solution (0.1mL, containing potassium hydroxide 0.6mg). Move to 80°C, react for 12h, cool the reaction solution to room temperature, add water (1mL) to the Schlenk tube, extract with ethyl acetate (2mL×3), evaporate the solvent; silica gel column chromatography (petroleum ether / ethyl acetate Ester = 10 / 1, v / v) to obtain 55.6 mg of a white solid (Intermediate IIIa), with a yield of 96% and an ee value of 96%.
[0049] The relevant identification data of intermediate IIIa are as follows:
[0050] [α] D 20 =+1.1×10 2...
Embodiment 2
[0071]
[0072] Ethyl 3-(2-pyridyl)acrylate (35.4 mg, 0.20 mmol), 4-bromophenylboronic acid (compound IIb, 80.3 mg, 0.40 mmol), chiral rhodium catalyst [RhCl(L3)] 2 (1.5mg, 0.20mmol%) was added to a Schlenk tube, under the protection of high-purity nitrogen, tetrahydrofuran (1mL) and potassium hydroxide aqueous solution (0.1mL, containing 0.6mg potassium hydroxide) were sequentially added. Move to 80°C, react for 12h, cool the reaction solution to room temperature, add water (1mL) to the Schlenk tube, extract with ethyl acetate (2mL×3), evaporate the solvent; silica gel column chromatography (petroleum ether / ethyl acetate Ester = 10 / 1, v / v) to obtain 61.5 mg of a white solid (intermediate IIIb), with a yield of 92% and an ee value of 96%.
[0073] The relevant identification data of compound IIIb are as follows:
[0074] [α] D 20 =+93 (c 0.50, CHCl 3 ).
[0075] ESI-MS(m / z):334.0449.
[0076] 1 H NMR (500MHz, CDCl 3 )δ8.59(d, J=4.5Hz, 1H), 7.59(td, J=7.7, 1.6Hz, 1H)...
Embodiment 3
[0095]
[0096] Ethyl 3-(2-pyridyl)acrylate (35.4mg, 0.20mmol), 4-chlorophenylboronic acid (48.8mg, 0.40mmol), chiral rhodium catalyst [RhCl(L3)] 2 (1.5mg, 0.20mmol%) was added to the Schlenk tube, pumped, and then added toluene (1mL) and potassium hydroxide aqueous solution (0.1mL, containing 0.6mg potassium hydroxide) under the protection of high-purity nitrogen. Move to 80°C, react for 12 hours, cool the reaction solution to room temperature, add water (1 mL) to the Schlenk tube, extract with ethyl acetate (2 mL×3), evaporate the solvent to obtain intermediate IIIa. After silica gel column chromatography (petroleum ether / ethyl acetate=10 / 1, v / v), 53.9 mg of a white solid was obtained, with a yield of 93% and an ee value of 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com