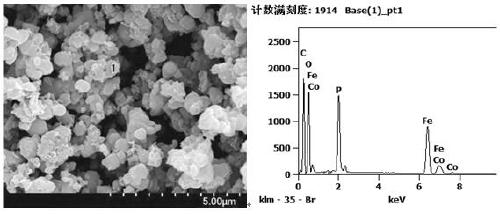
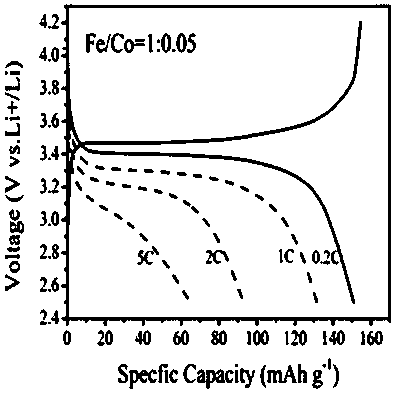
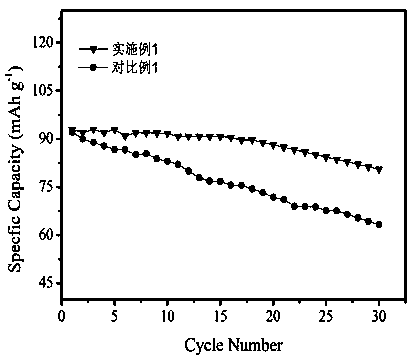
Preparation method of metal-doped lithium iron phosphate
A lithium iron phosphate, metal technology, applied in the field of lithium ion battery cathode materials, can solve the problems of poor cycle performance and mechanical processing performance, and achieve the effects of good mechanical processing performance and rate performance, high volume energy density and cycle performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A preparation method of doped metal lithium iron phosphate, the specific steps are as follows:
[0025] (1) Preparation of precursor: Add iron source (mixed with ferrous sulfate and ferrous nitrate at a mass ratio of 1:1) and phosphorus source (mixed with ammonium dihydrogen phosphate and diammonium hydrogen phosphate at a mass ratio of 1:1) into water to prepare Mixed solution, the concentration of iron in the mixed solution is 0.1mol / L, the molar ratio of iron and phosphorus is Fe:P=1:1, the mixed solution is stirred vigorously and then added hydrogen peroxide to obtain flocculent precipitation, hydrogen peroxide and Molar ratio H of iron element in iron source 2 o 2 : Fe=0.6:1, filter after stirring for 4 hours, wash the precipitate with deionized water repeatedly for residual impurities, and obtain the amorphous iron phosphate precursor after drying;
[0026] (2) Precursor intercalation of lithium source: disperse lithium source (lithium carbonate, lithium hydroxi...
Embodiment 2
[0033] A preparation method of doped metal lithium iron phosphate, the specific steps are as follows:
[0034] (1) Preparation of precursor: add iron source (ferrous chloride) and phosphorus source (diammonium hydrogen phosphate) into water to form a mixed solution. The concentration of iron in the mixed solution is 1mol / L, and the molar ratio of iron to phosphorus It is Fe:P=0.8:1.2, the mixed solution is stirred vigorously and then added hydrogen peroxide to obtain flocculent precipitation, the molar ratio H of hydrogen peroxide to iron element in the iron source 2 o 2 : Fe=0.8:1, after stirring for 8 hours, filter, rinse the precipitate with deionized water repeatedly for residual impurities, and obtain the amorphous iron phosphate precursor after drying;
[0035] (2) Precursor intercalation of lithium source: disperse lithium source (lithium acetate) and reducing agent tartaric acid into organic solvent ethanol to obtain an organic solution, the molar ratio of lithium sou...
Embodiment 3
[0038] A preparation method of doped metal lithium iron phosphate, the specific steps are as follows:
[0039] (1) Preparation of precursor: add iron source (ferrous oxalate) and phosphorus source (diammonium hydrogen phosphate) into water to form a mixed solution, the concentration of iron in the mixed solution is 2mol / L, and the molar ratio of iron and phosphorus is Fe :P=1.2:0.8, after the mixed solution is vigorously stirred, hydrogen peroxide is added to obtain a flocculent precipitate, and the molar ratio of hydrogen peroxide to iron in the iron source is H 2 o 2:Fe=1:1, after stirring for 12 hours, filter, wash the precipitate with deionized water repeatedly to remove residual impurities, and dry to obtain the amorphous iron phosphate precursor;
[0040] (2) Precursor intercalation of lithium source: disperse lithium source (lithium nitrate) and reducing agent ascorbic acid into the organic solvent propanol to obtain an organic solution, the molar ratio of lithium sour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com