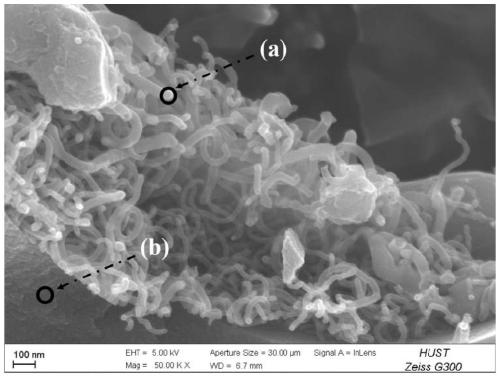
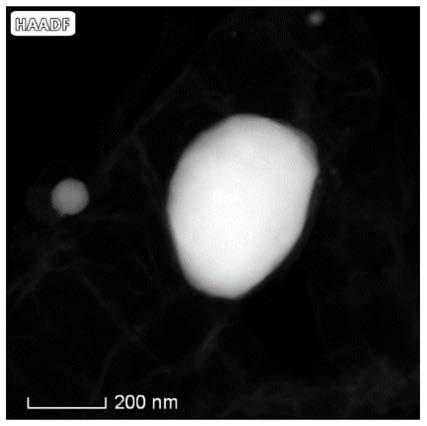
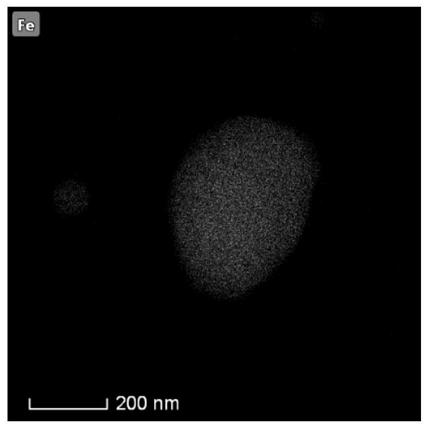
Method for preparing oxygen reduction catalyst from biomass and product
A biomass and catalyst technology, which is applied in the field of biomass-based carbon material preparation, can solve problems such as unstable catalytic activity, difficult quantitative control of nitrogen content, and difficulties in conductivity and active site technology.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) Wash and crush the collected chestnut shells to 80 mesh, dry in an oven at 80 degrees for 24 hours, take 10 g of dried samples, add 10 ml of 0.1 mol / l ferric nitrate solution (that is, 1 g of chestnut shells corresponds to 0.01 mol of nitric acid iron), stirred at room temperature for 24 hours, and vacuum freeze-dried at minus 30°C;
[0055] (2) Use a fixed-bed reactor with a diameter of 45 mm and a length of 60 mm to pyrolyze the impregnated sample. After the reactor is heated to 700 ° C, the sample is quickly placed in the middle of the reactor (the heating rate is 20 ° C / min ), the reaction time is 30min, the mixture is fully pyrolyzed, the argon gas flow rate is 50min / min, and the pyrolytic carbon is obtained by cooling under an inert atmosphere, which is recorded as composite material-1;
[0056] (3) Take 1g of composite material-1, disperse it into 50ml of ethanol solution, soak and stir for 12h, add 10g of melamine into the solution soaked in composite mate...
Embodiment 2
[0061] (1) Wash and crush the collected bamboo chips to 120 mesh, dry in an oven at 80 degrees for 24 hours, take 10 g of the dried sample, add 500 ml of 0.2 mol / l cobalt oxalate solution, stir at room temperature for 24 hours, minus 40 ℃ vacuum freeze-drying;
[0062] (2) Use a fixed-bed reactor with a diameter of 45mm and a length of 60mm to pyrolyze the impregnated sample. After heating the reactor to 900°C, put the sample into the middle of the reactor quickly, and the reaction time is 300min. Fully pyrolyze, the helium flow rate is 50min / min, and cool in an inert atmosphere to obtain pyrolytic carbon, which is recorded as composite material-1;
[0063](3) Take 1g of composite material-1, disperse it into 200ml of isopropanol solution, soak and stir for 24 hours, add 20g of melamine into the solution soaked in composite material-1, and add 200ml of 0.2mol / l manganese acetate solution at the same time, shake, Stir for 5 hours, dry in an oven at 80°C for 36 hours to obtain ...
Embodiment 3
[0068] (1) Wash and crush the collected bamboo chips to 120 mesh, dry in an oven at 80 degrees for 24 hours, take 10 g of the dried sample, add 200 ml of 0.1 mol / l nickel nitrate solution, stir at room temperature for 24 hours, minus 50 ℃ vacuum freeze-drying to obtain the impregnated sample;
[0069] (2) Use a fixed-bed reactor with a diameter of 45 mm and a length of 60 mm to pyrolyze the impregnated sample. After heating the reactor to 1000 ° C, the sample is quickly placed in the middle of the reactor, and the reaction time is 300 min. Fully pyrolyze, the argon flow rate is 50min / min, and cool in an inert atmosphere to obtain pyrolytic carbon, which is recorded as composite material-1;
[0070] (3) Take 1g of composite material-1, disperse it into 100ml of ethanol solution, soak and stir for 12h, add 5g of melamine into the solution soaked in composite material-1, and add 500ml of manganese sulfate solution of 0.05mol / l at the same time, oscillate, and stir for 24h , drie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com