Method for preparing coix seed oil composite nanoparticles
A technology of Coix seed oil and composite granules, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, and drug combinations, etc. Long time and other problems, to achieve the effect of improving digestion resistance and targeted absorption, low production cost, and reducing preparation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A high-efficiency preparation method of coix seed oil nanocomposite particle carrier, comprising the following steps:
[0033] (1) Dispersing wheat gliadin (Gliadin) in 60% (V / V) ethanol solution, until the mass fraction of gliadin in the solution is 1%, until it is completely dissolved;
[0034] (2) Coix seed oil (CSO) was added at a mass ratio of 5:1 to prolamin, and magnetically stirred until the coix seed oil was completely dispersed and dissolved to obtain a dispersion;
[0035] (3) Pour the carboxymethyl chitosan (CMCS) solution into the vortex-stirred dispersion until the mass fraction of carboxymethyl chitosan in the dispersion is 0.5%, adjust the pH value to 4, stir for 10 min, and rotate Evaporate for 10min to remove ethanol;
[0036] (4) Add calcium ion solution until the mass fraction of calcium ion in the reaction system is 0.5%, stir for 10min,
[0037] Coix seed oil-prolamin-carboxymethyl chitosan-calcium ion colloid solution was prepared.
[0038] (5)...
Embodiment 2-5
[0040] Polysaccharide is carboxymethyl chitosan, and add-on is respectively 1mg (accounting for dispersion liquid mass fraction 0.1%) (embodiment 2), 2mg (accounting for dispersion liquid mass fraction 0.2%) (embodiment 3), 3mg (accounting for dispersion liquid mass fraction 0.2%) (embodiment 3), 3mg (accounting for dispersion liquid mass fraction 0.3% by mass fraction) (Example 4), 4 mg (accounting for 0.4% by mass fraction of the dispersion liquid) (Example 5), calcium ions accounted for 0.5% by mass fraction of the reaction system.
[0041] Other steps and raw materials are with embodiment 1.
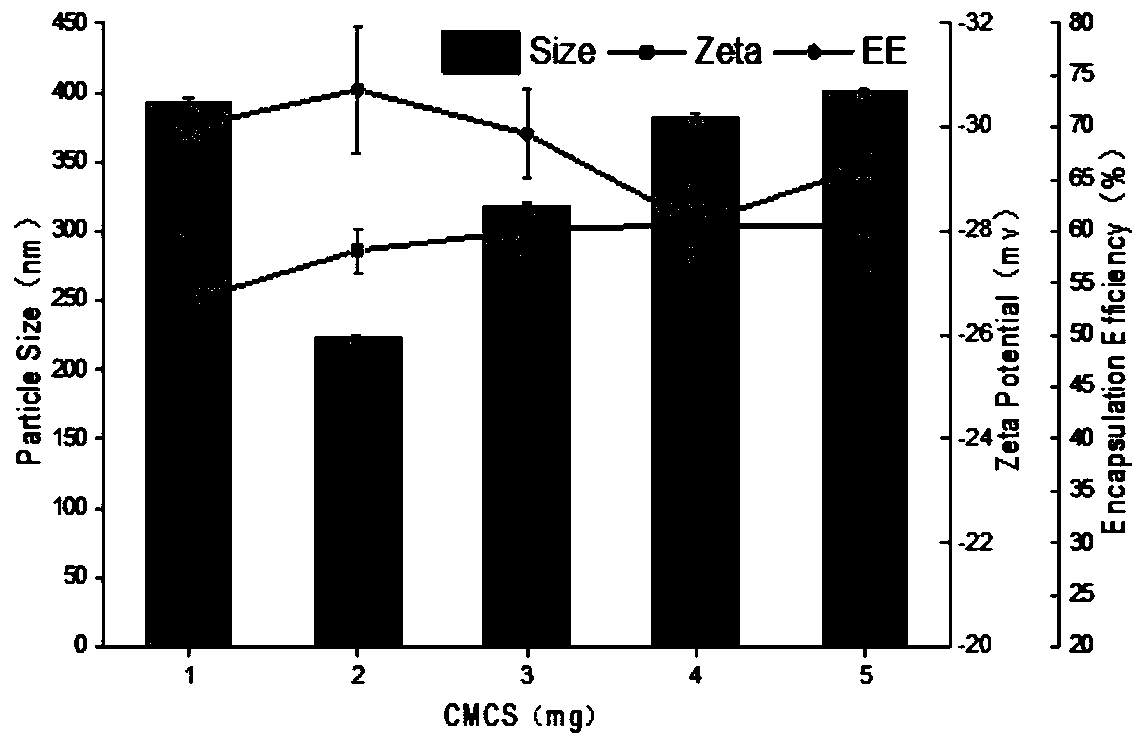
[0042] The particle diameter (Size), surface potential (Zeta) and embedding efficiency (EE) of above embodiment gained nanoparticle are as follows figure 1 shown. It can be seen that the coix seed oil-prolamin-carboxymethyl chitosan nanoparticles obtained in each embodiment maintain good particle size and potential, and the embedding rate is >50%. When the mass fraction of carboxym...
Embodiment 6-11
[0044] Calcium ion is as cation, and its consumption is respectively 0mg (embodiment 6), 0.05mg (accounting for reaction system mass fraction is 0.05%) (embodiment 7), 0.1mg (accounting for reaction system mass fraction is 0.1%) (embodiment 8 ), 0.2mg (accounting for reaction system mass fraction is 0.2%) (embodiment 9), 0.3mg (accounting for reaction system mass fraction is 0.3%) (embodiment 10), 0.4mg (accounting for reaction system mass fraction is 0.4%) (Example 11). The mass fraction of carboxymethyl chitosan in the dispersion is 0.2%.
[0045] Other steps and raw materials are with embodiment 1.
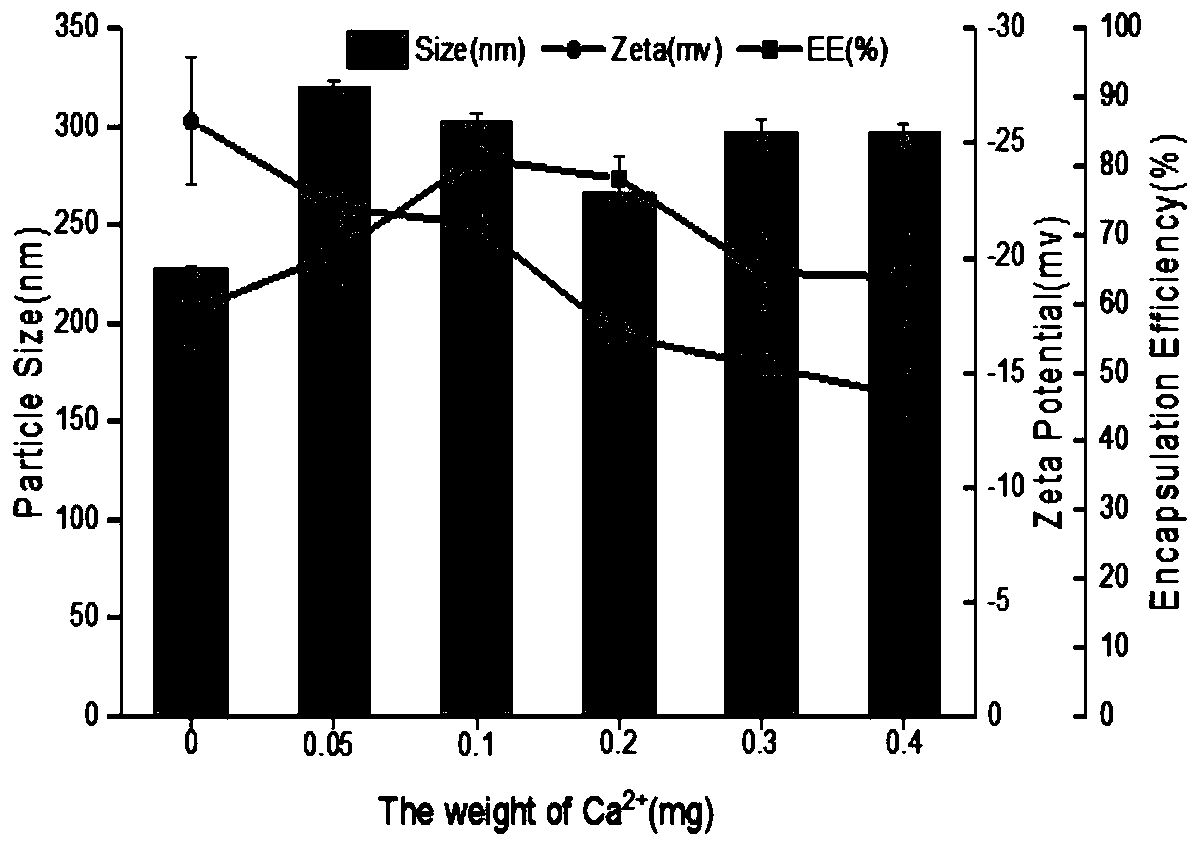
[0046] The particle diameter (Size), surface potential (Zeta) and embedding efficiency (EE) of above embodiment gained nanoparticle are as follows figure 2 shown. It can be seen that the coix seed oil-prolamin-carboxymethyl chitosan nanoparticles obtained in each embodiment maintain good particle size and potential, and the embedding rate is >50%. When the mass fraction of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| surface potential | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com