Fluorescent probe for selectively detecting singlet oxygen in solution phase and application
A singlet oxygen and fluorescent probe technology, applied in the field of fluorescent probes, can solve problems such as photobleaching and interference detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 (synthesis of probe):
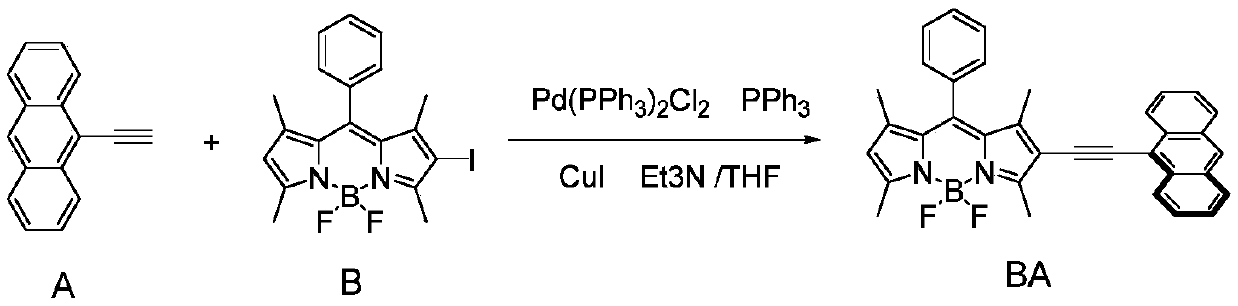
[0027] Such as image 3 As shown, the structure of the probe compound used in the examples is represented by the code BA, and the boron pyrrole precursor used in the synthesis of the probe compound is represented by the code B.
[0028] Synthesis of BA: Under nitrogen protection, B (0.09g, 0.2mmol) and A (0.085g, 0.42mmol) were dissolved in a mixed solvent of 5ml of anhydrous triethylamine and 10ml of anhydrous tetrahydrofuran, and Pd(PPh 3 ) 2 Cl 2 (14mg, 0.02mmol), PPh 3 (10.5mg, 0.04mmol), CuI (7.6mg, 0.04) and stirred at reflux for 8 hours. The solvent was evaporated under vacuum, and the obtained solid was purified by silica gel column chromatography to obtain the target compound BA.
[0029] 1 H NMR (400MHz, CDCl 3 )δ(ppm):8.57-8.55(d,2H),8.40(s,1H),8.01-7.99(d,2H),7.57-7.47(m,7H),7.36-7.34(m,2H),6.07 (s,1H),2.91(s,3H),2.62(s,3H),1.72(s,3H),1.43(s,3H).ESI-HRMS):m / z, Calcd for[M+H] + C 35 h 28 BYZGR 2 + :525.2308,foun...
Embodiment 2
[0030] Embodiment 2 (BA produces to photosensitizer MB illumination 1 o 2 quantitative detection):
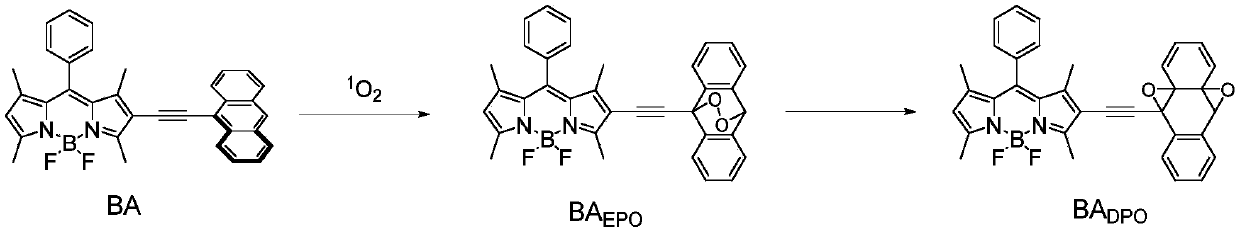
[0031] Add 3.0ml of 10μM BA and 10μM MB air-saturated dichloromethane / acetonitrile (v / v=1:1) mixed solution into a quartz cuvette, shake well, then add a 650nm high-pass filter in front of the xenon lamp, power About 0.2mW. Shake the solution evenly after a certain period of light, and measure the absorption spectrum and fluorescence spectrum. 1 o 2 Schematic diagram of yield variation.
[0032] Figure 5(a) shows the change of the absorption intensity of the system, indicating that with 1 o 2 As production increases, BA absorption intensity decreases while BA EPO The absorption intensity rises, and the absorption spectrum shifts blue; Figure 5(b) shows the linear fitting curve of the 518nm / 545nm absorbance ratio with the illumination time, and the linear regression constant of the linear fitting curve is 0.9991, indicating that the probe can quantitatively determine the ...
Embodiment 3
[0033] Embodiment 3 (BA is to 1 o 2 optional):
[0034] Add 2.0ml of 10μM BA air-saturated dichloromethane / acetonitrile (v / v=1:1) mixed solution into a quartz cuvette, then add 300.0μM oxidizing substances (oxidizing substances include hydrogen peroxide, hypochlorite, single line Oxygen, tert-butanol peroxide, nitrosyl peroxide (ONOO - )) in ethanol solution 1.0ml, shake the solution, equilibrate for 1h, measure the fluorescence spectrum. Add 1.0ml of ethanol as a control group. Figure 6 Indicates the change in fluorescence of the system after adding oxidative compounds to probe BA, it can be seen that probe BA is 1 o 2 Be selective.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com